|
Foraging Tactics and Trophic Ecology © Eric R. Pianka Optimal Foraging Theory Earnest enquiries into animal foraging behaviors began 45 years ago with publication of three important papers (MacArthur and Pianka 1966, Emlen 1966, and Pianka 1966). The first two papers by MacArthur and Pianka (1966) and Emlen (1966) were theoretical, examining the logic of animal feeding behaviours by identifying benefits and costs associated with various activities. These seminal papers ushered in the field now known as optimal foraging theory (OFT). In their paper entitled "On Optimal Use of a Patchy Environment," MacArthur and Pianka (1966) developed a graphical model of animal feeding activities based on costs versus profits. A forager's optimal diet can be specified and some interesting predictions emerge. Prey abundance influences the degree to which a consumer can afford to be selective because if affects search time per item eaten. MacArthur and Pianka made a robust prediction: Diets should be broad when prey are scarce (long search time), but narrow if food is abundant (short search time) because a consumer can afford to bypass inferior prey only when there is a reasonably high probability of encountering a superior item in the time it would have taken to capture and handle the previous one. Also larger patches should be used in a more specialized way than smaller patches because travel time between patches (per item eaten) is lower. 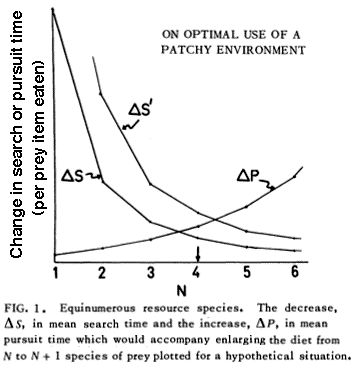
In an environment with a scant food supply, a consumer presumably cannot afford to bypass many inferior prey items because mean search time per item encountered is long and expectation of prey encounter is low (MacArthur and Pianka 1966). In such an environment, a broad diet maximizes returns per unit expenditure, favoring generalization. In a food-rich environment, however, search time per item is low because a foraging animal encounters numerous potential prey items. Under such circumstances, substandard prey items can be bypassed economically because expectation of finding a superior item in the near future is high. Hence rich food supplies favor selective foraging and lead to narrow food niche breadths. This is one of the most robust theorems of optimal foraging theory. These arguments are supported by the North American teiid lizard Aspidoscelis (formerly Cnemidophorus) tigris, which eats a greater diversity of foods in drier than average years (presumably times of low food availability) but like most lizards contracts its diet during periods of prey abundance (Pianka 1986, see following figure). Behavioral ecologists quickly embraced OFT because it conferred apparent rigor and generated testable predictions in a sometimes subjective field. Theoreticians explored a variety of ways to employ OFT, which became a minor growth industry during the 1970s and early 1980s. Studies of foraging behavior have proliferated extensively over the past three decades and several books have been written about foraging (Kamil and Sargent 1982; Stephens and Krebs 1986; Kamil, Krebs, and Pulliam 1987; Reilly, McBrayer, and Miles 2007). Pianka (1966) also adopted a more descriptive empirical approach based on his observations of desert lizards, identifying two distinct modes of foraging: 'sit-and-wait' (ambush predators) versus 'widely-foraging' (more active predators). Of course, this dichotomy could be somewhat artificial, although numerous animal groups seem to fall rather naturally into either one category or the other. Members of most lizard families typically exploit either one or the other of these two modes of foraging: thus iguanians, agamids and geckos primarily sit-and-wait for their prey, whereas teiids, skinks, and varanids forage widely. Lacertids, however, exploit both modes of foraging, even within the same genus. This evidently natural dichotomy in foraging tactics has had a substantial impact on theories of optimal diets and competitive relationships among species (Magnusson et al. 1985; McLaughlin 1989; Perry et al. 1990; Pietruszka 1986; Reilly, McBrayer, and Miles 2007). Certain dietary differences are associated with this apparent dichotomy in foraging tactics. Sit-and-wait predators rely largely on moving prey whereas widely-foraging predators encounter and consume non-moving types of prey items more frequently. For the sit-and-wait tactic to pay off, prey must be relatively mobile and prey density must be high (or predator energy requirements low). The sit-and-wait tactic should be less prevalent during periods of prey scarcity than the widely-foraging method. The success of the widely-foraging tactic is also influenced by prey mobility and prey density as well as by the predator's energetic requirements (which should usually be higher than those of sit-and-wait predators), but the searching abilities of the predator and the spatial distribution of its prey now assume substantial importance. After heavy summer rains, when termites send out their winged reproductives in great abundance, virtually every species of lizard eats nothing but termites (even lizard species that normally never consume termites). During such fleeting moments of extraordinary prey abundance, competition for food is negligible and dietary overlap among members of a desert saurofauna is sometimes nearly complete. Another important spin-off of foraging mode involves reproductive tactics. Clutch sizes of widely-foraging species are smaller than those of sit-and-wait species, probably because the former draw the attention of predators by their constant movements and simply cannot afford to weight themselves down with eggs to as great an extent as can the latter. Hence foraging style constrains reproductive prospects (as well as vice versa). Huey and Pianka (1981) summarized many of these ecological correlates of foraging mode in the following table. So-called 'optimal foraging theory' and Pianka's 'modes of foraging' constitute two distinct approaches to foraging that have evolved and begun to coalesce during the past decade. The literature on animal foraging was reviewed in Trends in Ecology and Evolution by Perry and Pianka (1997), and elevated to a paradigm in a 2007 book 'Lizard Ecology: The Evolutionary Consequences of Foraging Mode' (Reilly, L. D. McBrayer, and D. Miles, eds.) with an introduction by Huey and Pianka and a chapter by Vitt and Pianka. Vitt and Pianka have also examined the ambush-active foraging mode dichotomy from a phylogenetic perspective and used it in a major synthesis explaining lizard evolution in their Lizards book and several papers, including a 2005 PNAS paper. The emerging new framework is more complex than originally thought, combining both theory and observation. Modern phylogenetic methods promise powerful new insights into how animal foraging has evolved.  Australian termite specialist, Diplodactylus pulcher. Ancestors of squamate reptiles (lizards and snakes) were diapsids with two temporal fenestrae and rigid skulls like those of the tuatara. An important evolutionary innovation and a major derived characteristic that defines squamates is streptostyly, the loss of the lower temporal arch. Only the upper aperture is retained by squamates, and some lizards and snakes have lost both arches. This freed the quadrate to move and resulted in an efficient new hanging jaw mechanism. Streptostyly increased gape and speed of jaw closure. With evolution of new musculature, this new jaw mechanism also increased mechanical advantage and biting force. Streptostyly was probably the "key innovation" that gave squamates access to new food resources, as well as enabling them to diversify by developing new feeding strategies and a wide variety of dietary specializations. Ancestral squamates were ambush predators, detected prey by movement using visual cues, captured prey with their tongues, had relatively low activity levels, and had poorly developed chemosensory systems. All these features have been retained in members of the large clade Iguania (Iguanids, Agamids, and Chameleons). Chameleons have taken lingual feeding to its logical extreme with their ballistic tongues. 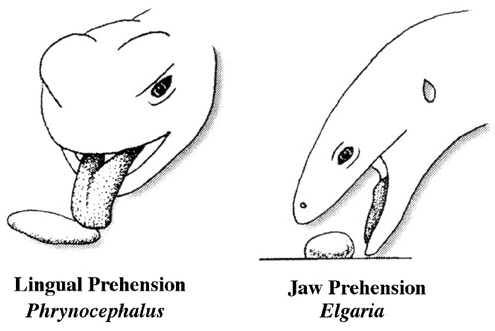 By far, one of the most important junctures in squamate evolution occurred when an ancestor
switched from tongue prehension of prey to jaw prehension (Schwenk and Throckmorton, 1989).
This freed the tongue from its role in prey capture, allowing it to
be used as a chemical sampler, carrying non-airborne particles into the mouth to be received
and deciphered by the vomeronasal system. The vomeronasal system was present in squamate
ancestors, but with few exceptions, has remained rudimentary in iguanians. The foretongue and
hyobranchium became uncoupled, the foretongue achieved greater and greater specialization
for picking up and transporting chemical signals, and the skull became less robust and more
kinetic than iguanian skulls. The temporal region is less broad due to reduction or loss of
the upper temporal fenestra. Autarchoglossans have two additional points of potential
flexibility in their skulls, a condition known as cranial kinesis or mesokinesis.
By far, one of the most important junctures in squamate evolution occurred when an ancestor
switched from tongue prehension of prey to jaw prehension (Schwenk and Throckmorton, 1989).
This freed the tongue from its role in prey capture, allowing it to
be used as a chemical sampler, carrying non-airborne particles into the mouth to be received
and deciphered by the vomeronasal system. The vomeronasal system was present in squamate
ancestors, but with few exceptions, has remained rudimentary in iguanians. The foretongue and
hyobranchium became uncoupled, the foretongue achieved greater and greater specialization
for picking up and transporting chemical signals, and the skull became less robust and more
kinetic than iguanian skulls. The temporal region is less broad due to reduction or loss of
the upper temporal fenestra. Autarchoglossans have two additional points of potential
flexibility in their skulls, a condition known as cranial kinesis or mesokinesis.
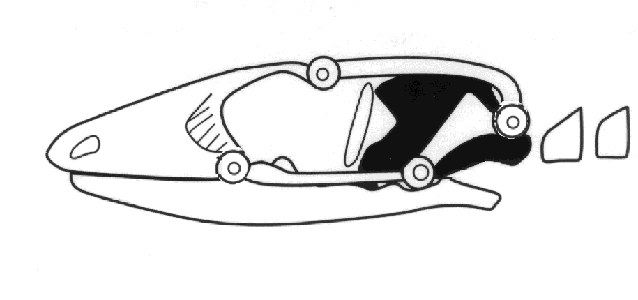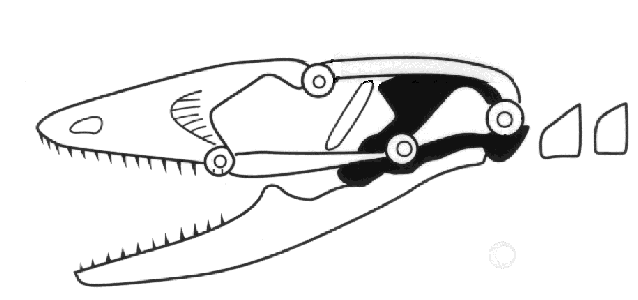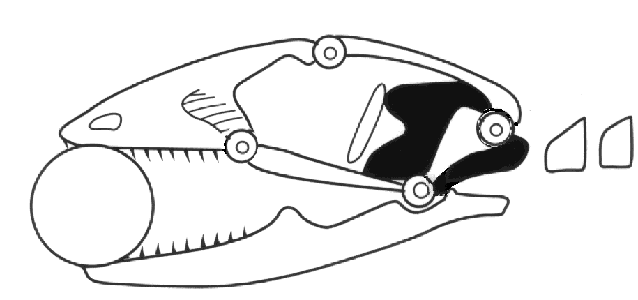 Mesokinesis allows the upper jaw to bend and better conform to prey, presumably enhancing
feeding success. These combined skull and chemosensory modifications gave autarchoglossans
access to microhabitats and prey unavailable to iguanians and likely predisposed them to
higher activity levels. For example, the ability to detect and discriminate prey chemically
allowed autarchoglossans access to prey that could not be detected visually, including highly
cryptic arthropods and invertebrates and vertebrates living in crevices, in the ground, and
in some cases in water. They were no longer limited to prey moving across their field of
vision. Remaining in one place for long periods of time had a low energetic payoff compared
to actively searching through the habitat for prey that need not be seen. Active or wide
foraging provided these lizards a competitive advantage and selected for higher levels of
activity. Moving about searching for prey also has its costs, both energetically and via
increased exposure to potential predators. Widely-foraging autarchoglossans find and consume
more prey calories per unit time than do iguanians (Huey and Pianka, 1981).
Mesokinesis allows the upper jaw to bend and better conform to prey, presumably enhancing
feeding success. These combined skull and chemosensory modifications gave autarchoglossans
access to microhabitats and prey unavailable to iguanians and likely predisposed them to
higher activity levels. For example, the ability to detect and discriminate prey chemically
allowed autarchoglossans access to prey that could not be detected visually, including highly
cryptic arthropods and invertebrates and vertebrates living in crevices, in the ground, and
in some cases in water. They were no longer limited to prey moving across their field of
vision. Remaining in one place for long periods of time had a low energetic payoff compared
to actively searching through the habitat for prey that need not be seen. Active or wide
foraging provided these lizards a competitive advantage and selected for higher levels of
activity. Moving about searching for prey also has its costs, both energetically and via
increased exposure to potential predators. Widely-foraging autarchoglossans find and consume
more prey calories per unit time than do iguanians (Huey and Pianka, 1981).
North American and Australian sites support similar numbers of species of sit-and-wait foragers, whereas this mode of foraging is distinctly impoverished in the Kalahari (Pianka 1986). Markedly fewer species forage widely in western North America (only one species, the teiid Aspidoscelis (formerly Cnemidophorus) tigris) and in the Kalahari (an average of 4 species per site) than in the Australian deserts (mean number of widely-foraging species per area is 10.1, most of which are skinks in the genus Ctenotus). Intercontinental comparisons of proportions of total species in various foraging modes are also instructive: a full 60% of North American lizard species are sit-and-wait foragers, compared to only 16% in the Kalahari and 18% in Australia; percentages of widely-foraging species are 14% (North America), 27% (Kalahari), and 36% (Australia). These differences stem from historical contingency. Two species of Kalahari lizards, Pedioplanis lineoocellata and Meroles suborbitalis, sit-and-wait for prey, whereas two other syntopic species, Heliobolus lugubris and Pedioplanis namaquensis), forage widely for their food (Pianka et al. 1979; Huey and Pianka 1981). Time budgets of these lacertids reflect their modes of foraging (Pianka 1986). Foraging widely is energetically expensive and, judging from their relative stomach volumes, those species that engage in this mode of food gathering appear to capture more prey per unit time than do sit-and-wait species. Overall energy budgets of widely-foraging species are nearly twice as great as those of sit-and-wait species (Huey and Pianka 1981). Sedentary foragers tend to encounter and eat fairly mobile prey whereas more active widely-foraging predators consume less active prey. Compared with sit-and-wait species, widely-foraging lacertid species eat more termites (sedentary, spatially and temporally unpredictable but clumped prey).  One widely-foraging species, Nucras tessellata, specializes on scorpions (by day,
these large arachnids are non-mobile and exceedingly patchily-distributed prey items).
Another ramification of foraging mode in these Kalahari lizards concerns exposure to their
own predators. Because of their more-or-less continual movements, widely-foraging species
expose themselves and tend to be more visible. As a result, they seem to suffer higher
predation rates. Widely-foraging species fall prey to lizard predators that hunt by ambush
whereas sit-and-wait lizard species tend to be eaten by predators that forage widely,
generating "crossovers" in foraging mode between trophic levels. Widely-foraging lizard
species are also more streamlined and have longer tails than sit-and-wait species (Huey
and Pianka 1981).
One widely-foraging species, Nucras tessellata, specializes on scorpions (by day,
these large arachnids are non-mobile and exceedingly patchily-distributed prey items).
Another ramification of foraging mode in these Kalahari lizards concerns exposure to their
own predators. Because of their more-or-less continual movements, widely-foraging species
expose themselves and tend to be more visible. As a result, they seem to suffer higher
predation rates. Widely-foraging species fall prey to lizard predators that hunt by ambush
whereas sit-and-wait lizard species tend to be eaten by predators that forage widely,
generating "crossovers" in foraging mode between trophic levels. Widely-foraging lizard
species are also more streamlined and have longer tails than sit-and-wait species (Huey
and Pianka 1981).
Lizard foraging mode is strongly constrained by phylogeny (Cooper 1994; Perry 1995; Vitt et al. 2003). Virtually all iguanians are sedentary ambush foragers, whereas most autarcho-glossans are active foragers. Lizards in the old world family Lacertidae constitute an exception, however, exhibiting both modes of foraging (Huey and Pianka 1982). These studies demonstrate the importance of phylogenetic comparative methods in studying animal foraging. Sucn an approach allows data to be interpreted in a historical, evolutionary perspective, and assumptions about the genetic bases of behavior can be tested. Most importantly, analyses can be performed that exclude effects of species relatedness, preventing historical pseudo-replication from affecting conclusions (Vitt and Pianka 2005). Certain species of lizards are dietary specialists, eating a narrow range of prey types. For example, the Australian pygopodid Pygopus nigriceps and the Kalahari lacertid Nucras tessellata both consume an excessive number of scorpions when compared to other lizard species (Pianka 1986). Nucras forages widely to capture these large arachnids by day in their diurnal retreats, whereas the nocturnal Pygopus sits and waits for scorpions at night above ground during the latter's normal period of activity. In North America, no desert lizard has become a scorpion specialist, but the small sand-swimming snake Chionactis occipitalis seems to have usurped this ecological role.  Scorpions are solitary prey items, but they are extremely large and nutritious, facilitating evolution of dietary specialization. For similar reasons, specialization on other lizards as food items has evolved in North American leopard lizards (Crotaphytus wislizeni) as well as among most Australian varanids. Other lizard species have much more catholic diets, eating a considerably wider variety of foods. Dietary niche breadth also varies within species from time to time and from place to place as the composition of diets change with opportunistic feeding in response to fluctuating prey abundances and availabilities (Pianka 1986). However, the consistency of lizard diets is remarkable, suggesting a profound impact of microhabitat utilization, foraging mode, as well as various anatomical and behavioral constraints imposed by phylogeny.  Meroles (formerly Aporosaura) anchietae, Namib desert lacertid with duckbilled snout.  Diets of nearly a hundred species of desert lizards were summarized by Pianka (1986) as percentages by volume of various foods in the overall diets of all specimens of each species on all study areas within each desert-lizard system. Close scrutiny of these data matrices reveals that some species specialize on scorpions, ants, termites, vertebrates, and/or on plants, whereas other species on each continent are much more generalized, eating an extremely wide variety of food categories. No centipedes were found in stomachs of North American lizards, and solpugids are not present in Australia. Food niche breadths, calculated using the reciprocal of Simpson's (1949) index of diversity, 1/½pi2, range from 1.06 to 6.53 (mean 4.07, st. dev. = 1.93, N = 11) among the 11 species of North American lizards, from 1.07 to 8.22 (mean 3.85, st. dev. = 2.09, N = 21) among 21 Kalahari species, and from 1.00 to 10.9 (mean 3.86, st. dev. = 2.28, N = 59) among the 60 Australian species (Figure 5). None of these intercontinental variations in food niche breadths are significantly different by t-tests. Estimates of food niche breadths are uncorrelated with the number of lizards on which they are based ( r = 0.11), providing evidence that sample sizes are adequate to characterize patterns of food utilization even among the rarer species. Indeed, species with broad diets are often, though not always, relatively uncommon. Across species, dietary niche breadth is weakly, but significantly (r = .27, P < .02), positively correlated with microhabitat niche breadth, an indication that food specialists tend to be restricted to fewer microhabitats than food generalists. Some lizard species such as lacertids, however, are dietary specialists but microhabitat generalists (still others are microhabit specialists and dietary generalists).  Biologically significant variation between species in utilization of certain relatively minor
food categories is evident: for example, in the diets of climbing lizard species,
hemiptera-homoptera and mantids-phasmids as well as various flying insects (wasps,
Diptera, and Lepidoptera) tend to be better represented than they are among terrestrial
species. Likewise, geckos consume more nocturnal arthropods (scorpions, crickets, roaches,
and moths) than do most diurnal species (some diurnal lizards such as Nucras do
capture nocturnal prey in their diurnal retreats). Such prey items are thus indicators of
spatial and temporal patterns of activity.
Biologically significant variation between species in utilization of certain relatively minor
food categories is evident: for example, in the diets of climbing lizard species,
hemiptera-homoptera and mantids-phasmids as well as various flying insects (wasps,
Diptera, and Lepidoptera) tend to be better represented than they are among terrestrial
species. Likewise, geckos consume more nocturnal arthropods (scorpions, crickets, roaches,
and moths) than do most diurnal species (some diurnal lizards such as Nucras do
capture nocturnal prey in their diurnal retreats). Such prey items are thus indicators of
spatial and temporal patterns of activity.
Australian arboreal gecko, Diplodactylus ciliaris. Relatively few foods dominate lizard diets. Prey resource spectra are broadly similar between continents (Pianka 1986), although notable differences occur. In North America, the seven most important (totalling 84%), in decreasing order by volumetric importance, are: beetles, termites, insect larvae, grasshoppers plus crickets, ants, plant materials, and vertebrates. In the Kalahari, just three food categories far outweigh all others (total 71%): these are termites, beetles, and ants. In Australia, the five most important categories (total 77%, in decreasing order) are: vertebrates, termites, ants, grasshoppers plus crickets, and beetles. The same three categories, termites, beetles and ants, constitute major prey items in all three continental desert-lizard systems. Termites assume a disproportionate role in the Kalahari, as do vertebrate foods in Australia (largely a reflection of varanid diets). Somewhat surprisingly, the overall diversity of foods consumed by all species of lizards is actually greatest in the least diverse North American saurofauna (8.7), lowest in the Kalahari lizards (4.4), and intermediate in Australia (6.6). Basically comparable figures, although broadly overlapping, emerge from an area-by-area analysis (Pianka 1986, 1989). Prey diversity is weakly correlated with certain measures of the variability in average annual precipitation: food diversity is positively correlated with the coefficient of variation in annual precipitation (r = .45, P < .05), but is negatively correlated with the mean minus the standard deviation in precipitation. More variable precipitation, and presumably primary productivity, fosters higher insect species diversities (lizard diversity also correlates with variability of precipitation). Any given organism possesses a unique coadapted complex of physiological, behavioral, and ecological traits whose functions complement one another and enhance that organism's reproductive success. Such a constellation of adaptations has been called an optimal design (Rosen, 1967) or an adaptive suite (Bartholomew, 1972). 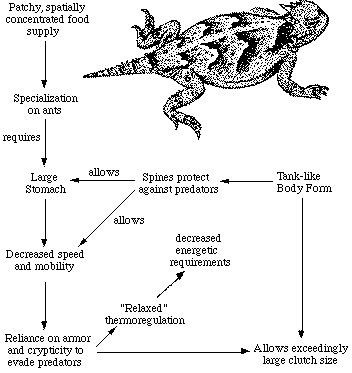 The adaptive suite of anatomical, behavioral, and ecological factors influ- encing an ant specialist, the desert horned lizard Phrynosoma platyrhinos. Consider the desert horned lizard Phrynosoma platyrhinos (above Figure). Various features of its anatomy, behavior, diet, temporal pattern of activity, thermoregulation, reproductive tactics, and predator escape tactics, can be profitably interrelated and interpreted to provide an integrated view of the ecology of this interesting animal (Pianka 1966; Pianka and Parker, 1975). Horned lizards are ant specialists and usually eat essentially nothing else. Ants are small and contain much undigestible chitin, so that large numbers of them must be consumed. An ant specialist must therefore possess a large stomach for its body size. When expressed as a proportion of total body weight, the stomach of this horned lizard occupies a considerably larger fraction of the animal's overall body mass (about 13 percent) than do the stomachs of all other sympatric desert lizard species, including the herbivorous desert iguana Dipsosaurus dorsalis (herbivores typically have lower assimilation rates and larger stomachs than carnivores). Possession of such a large gut necessitates a tanklike body form, reducing speed and decreasing the lizard's ability to escape from predators by flight. As a result, natural selection has favored a spiny body form and cryptic behavior rather than a sleek body and rapid movement to cover (as in most other species of lizards). Risks of predation are likely to be increased during long periods of exposure while foraging in the open. A reluctance to move, even when actually threatened by a potential predator, could well be advantageous; movement might attract attention of predators and negate the advantage of concealing coloration and contour. Such decreased movement doubtless contributes to the observed high variance in body temperature of Phrynosoma platyrhinos, which is significantly greater than that of all other species of sympatric lizards. Phrynosoma platyrhinos are also active over a longer time interval than any sympatric lizard species. Wide fluctuations in horned lizard body temperatures under natural conditions presumably reflect both the long activity period and perhaps their reduced movements into or out of the sun and shade (most of these lizards are in the open sun when first sighted). More time is thus made available for activities such as feeding. A foraging anteater must spend considerable time feeding. Food specialization on ants is economically feasible only because these insects usually occur in a clumped spatial distribution and hence constitute a concentrated food supply. To make use of this patchy and spatially concentrated, but at the same time not overly nutritious, food supply, P. platyrhinos has evolved a unique constellation of adaptations that include a large stomach, spiny body form, an expanded period of activity, and "relaxed" thermoregulation (eurythermy). The high reproductive investment of adult horned lizards is probably also a simple and direct consequence of their robust body form (Pianka and Parker, 1975; Vitt and Congdon, 1978).  Lizards that must be able to move rapidly to escape predators, such as racerunners Aspodoscelis (formerly Cnemidophorus), would hardly be expected to weight themselves down with eggs to the same extent as animals like horned lizards that rely almost entirely upon spines and camouflage to avoid their enemies. References on Foraging Tactics Bartholomew, G. A. 1972. Body temperature and energy metabolism. Chapter 8 (pp. 298-368) in M. S. Gordon (ed.), Animal physiology: Principles and adaptations. Macmillan, New York. Charnov, E. L. 1973. Optimal foraging: Some theoretical explorations. Ph.D. dissertation. Univ. of Washington, Seattle. Charnov, E. L. 1976a. Optimal foraging: Attack strategy of a mantid. Amer. Natur. 110: 141-151. Charnov, E. L. 1976b. Optimal foraging: The marginal value theorem. Theoret. Pop. Biol. 9: 129-136. Cooper, W. E. 1994. Prey chemical discrimination, foraging mode, and phylogeny. Chapter 5 (pp. 95-116) in L. J. Vitt and E. R. Pianka (eds.) Lizard Ecology: Historical and Experimental Perspectives. Princeton Univ. Press. Emlen, J. M. 1966. The role of time and energy in food preference. Amer. Natur. 100:611-617. Huey, R. B. and E. R. Pianka. 1981. Ecological consequences of foraging mode. Ecology 62: 991-999. Download pdf Huey, R. B. and E. R. Pianka. 2007. On Widely Foraging for Kalahari Lizards. Preface, pp. 1-10 in S. M. Reilly, L. D. McBrayer, and D. B. Miles, eds. "Lizard Ecology: The Evolutionary Consequences of Foraging Mode." Cambridge University Press. Read on line Huey, R. B., E. R. Pianka, and L. J. Vitt. 2001. How often do lizards "run on empty?" Ecology 82: 1-7. Download pdf Kamil, A. C. and T.D. Sargent (eds.). 1982. Foraging behavior. Garland Press, N. Y. Kamil, A. C., J. R. Krebs, and H. R. Pulliam (eds.). 1987. Foraging behavior. Plenum Press, N. Y. Krebs, J. R. 1978. Optimal foraging: Decision rules for predators. Chapter 2 (pp. 23-63) in J.R. Krebs and N. B. Davies (eds.), Behavioral ecology: An evolutionary approach. Blackwell, Oxford. Krebs, J. R. and A. Kacelnik. 1991. Decision-making. Chapter 4 (pp. 105-136) in J. R. Krebs and N. B. Davies (eds.) Behavioural Ecology, Third Ed. Blackwell. MacArthur, R. H., and R. Levins. 1967. The limiting similarity, convergence, and divergence of coexisting species. Amer. Natur. 101: 377-385. MacArthur, R. H. and E. R. Pianka. 1966. On optimal use of a patchy environment. American Naturalist 100: 603-609. Download pdf Magnusson, W. E., L. J. Paiva, R. M. Rocha, C. R. Franke, L. A. Kasper, and A. P. Lima. 1985. The correlates of foraging mode in a community of Brazilian lizards. Herpetologica 41: 324-332. McLaughlin, R. L. 1989. Search modes of birds and lizards: evidence for alternative movement patterns. Am. Nat. 133: 654-670. Orians, G. H., and N. Pearson. 1979. On the theory of central place foraging. In D.J. Horn, R. Mitchell, and G. R. Stairs, (eds.), Analysis of ecological systems (pp. 155-177). Ohio State Univ. Press, Columbus. Perry, G. and E. R. Pianka. 1997. Animal foraging: past, present and future. Trends in Ecology and Evolution 12: 360-364. Download pdf Perry, G., I. Lampl, A. Lerner, D. Rothenstein, E. Shani, N. Sivan and Y. Werner. 1990. Foraging mode in lacertid lizards: variation and correlates. Amphibia-Reptilia 11: 373-384. Pianka, E. R. 1966. Convexity, desert lizards, and spatial heterogeneity. Ecology 47: 1055-1059. Download pdf Pianka, E. R. 1986. Ecology and Natural History of Desert Lizards. Analyses of the Ecological Niche and Community Structure. Princeton University Press, Princeton, New Jersey. Pianka, E. R. 1989. Desert lizard diversity: additional comments and some data. American Naturalist 134: 344-364. Pierce, G. J. and J. G. Ollason. 1987. Eight reasons why optimal foraging theory is a complete waste of time. Oikos 49: 111-118. Pietruszka, R. D. 1986. Search tactics of desert lizards: How polarized are they? Animal Behavior 34: 1742-1758. Pulliam, H. R. 1974. On the theory of optimal diets. Amer. Natur. 108: 59-74. Pyke, G. H. 1984. Optimal foraging theory: a critical review. Ann. Rev. Ecol. Syst. 15: 523-575. Pyke, G. H., H. R. Pulliam, and E. L. Charnov. 1977. Optimal foraging: a selective review of theory and tests. Quart. Rev. Biol. 52: 137-154. Reilly, S. M., L. D. McBrayer, and D. B.Miles, eds. 2007. Lizard Ecology: The Evolutionary Consequences of Foraging Mode. Cambridge University Press. Rosen, R. 1967. Optimality principles in biology. Plenum, New York. Schoener, T. W. 1969. Models of optimal size for solitary predators. Amer. Natur. 103: 277-313. Schoener, T. W. 1969. Optimal size and specialization in constant and fluctuating environments: An energy-time approach. Brookhaven Symp. Biol. 22:103-114. Schoener, T. W. 1971. Theory of feeding strategies. Annual Review of Ecology and Systematics 2: 369-404. Schwenk, K. 1994. Why snakes have forked tongues. Science 263:1573-1577. Schwenk, K. 1995. Of tongues and noses: Chemoreception in lizards and snakes. Trends Ecol. Evol. 10: 7-12. Schwenk, K. 2000. Feeding in lepidosaurs. In Feeding: form, function and evolution in tetrapod vertebrates. K. Schwenk, ed. pages 175-291. San Diego, California: Academic Press. Schwenk, K., and G. S. Throckmorton. 1989. Functional and evolutionary morphology of lingual feeding in squamate reptiles: phylogenetics and kinematics. J. Zool. Lond. 219: 153-175. Stephens, D. W. and J. R. Krebs. 1986. Foraging theory. Princeton University Press, Princeton, N.J. Toft, C. A. 1981. Feeding ecology of Panamanian litter anurans: patterns in diet and foraging mode. J. Herpetology 15: 139-144. Vitt, L. J. and J. D. Congdon. 1978. Body shape, reproductive effort, and relative clutch mass in lizards: Resolution of a paradox. Amer. Natur. 112: 595-608. Vitt, L. J., E. R. Pianka, W. E. Cooper, and K. Schwenk. 2003. History and the global ecology of squamate reptiles. American Naturalist 162: 44-60. Download pdf Vitt, L. J. and E. R. Pianka. 2005. Deep history impacts present day ecology and biodiversity. Proc. Nat. Acad Sci. 102:7877-7881. Download pdf Vitt, L. J. and E. R. Pianka. 2007. Feeding Ecology in the Natural World. Chapter 5, pp. 141-172 in S. M. Reilly, L. D. McBrayer, and D. B.Miles, eds. "Lizard Ecology: The Evolutionary Consequences of Foraging Mode." Cambridge University Press. Read on line Back to Herpetology Home Page Pianka lab page |