Species Diversity © Eric R. Pianka Introduction Species richness, relative abundance, equitability, rarity, species accumulation curves, and species diversity are all intimately intertwined. Species richness, also known as species density, is merely a simple count of the number of species present. Such species lists summarize assemblages in ecosystems, providing basic information necessary for describing and understanding guild structure, community ecology, and conservation biology. Relative abundances show how many individuals of each species are present (species accumulation curves are just the inverse of relative abundance curves). Equitability represents the degree to which species are equally abundant. Rare and uncommon species contribute more than abundant species to overall species diversity (also known as biodiversity), which combines richness with equitability. These will now be considered in greater detail. Species Richness Why does one community contain more species than another? Some complex communities, such as coral reefs and tropical rain forests, consist of many thousands of different plant and animal species, whereas other communities, such as desert communities, support perhaps only hundreds of species. The number of species often varies greatly even at a local level; thus, a homogenous open habitat often contains fewer species than does an adjacent more complex habitat (Inger and Colwell 1977). Indeed, different forest communities in the same general region usually vary in numbers as well as in types of plant and animal species (MacArthur and MacArthur 1961; MacArthur 1965, 1972). Reptile species richness increases with structural habitat complexity (Pianka 1966, 1967; Inger and Colwell 1977; Pianka 1977; Fischer et al. 2004). The number of species is referred to as species richness or species density (Gotelli and Colwell 2001). Gotelli and Colwell (2001) discuss procedures and pitfalls in the measurement and comparison of species richness. From 1962 through 2008, I have gathered and analyzed extensive data on ecological relationships of lizard faunas at some 32 desert study sites, which lie at roughly similar latitudes on three continents: the Great Basin, Mojave, and Sonoran deserts in western North America, the Kalahari desert in southern Africa, and the Great Victoria desert of Western Australia (Pianka 1986). A series of 10-12 representative flatland desert areas were selected for investigation on each continent. Study sites are homogeneous and continuous, extensive enough to facilitate sampling, and generally well suited for ecological analyses. Study areas vary in size from about half a square kilometer to several square kilometers. Study sites exhibit a variety of habitat types, ranging from simple vegetation to more complex structure. Australian deserts have about 22 lizard genera and 62 species (Pianka 1969, 1986), African deserts 13 genera and 22 species (Pianka 1971), and North American deserts 12 genera and 12 species (Pianka 1967). Relative Abundance 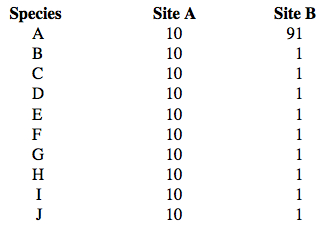 Communities with similar numbers of species often differ in yet another way. Some
contain a few very common species and many rare ones, whereas others support no
very common species but many of intermediate abundance. Suppose you are an avid bird
watcher and have a choice of going birding in two different areas, A or B. Each site
has ten bird species, but at site A all are equally abundant whereas at site B, most
are English sparrows and the other 9 species are all uncommon. If your goal was to see as
many species as possible, where would you choose to go?
Obviously you would be more likely to see a greater number of species at site A
where abundances are more equitable.
Communities with similar numbers of species often differ in yet another way. Some
contain a few very common species and many rare ones, whereas others support no
very common species but many of intermediate abundance. Suppose you are an avid bird
watcher and have a choice of going birding in two different areas, A or B. Each site
has ten bird species, but at site A all are equally abundant whereas at site B, most
are English sparrows and the other 9 species are all uncommon. If your goal was to see as
many species as possible, where would you choose to go?
Obviously you would be more likely to see a greater number of species at site A
where abundances are more equitable.
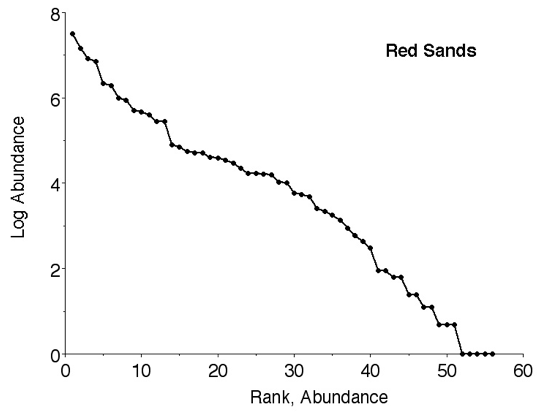
Abundance is only one way of estimating the relative importance of various component species within a community; other measures sometimes employed include both the biomass of and energy flow through various species' populations. The relative importance of species varies within and between communities, and considerable effort has been expended in attempts to document such differences and to understand why they occur. Importances of different species within a community (or a portion of one) can be conveniently depicted using relative abundance (Figures 1 and 2). 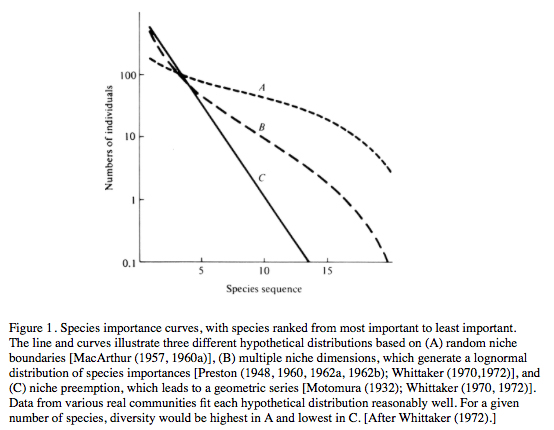
Several hypothetical distributions of the relative importance of species within communities have been suggested that generate different shaped curves of relative abundance (Motomura 1932; MacArthur 1960; Pianka 1973; Whittaker 1975; Magurran 1988; Pianka 2000, 2014). 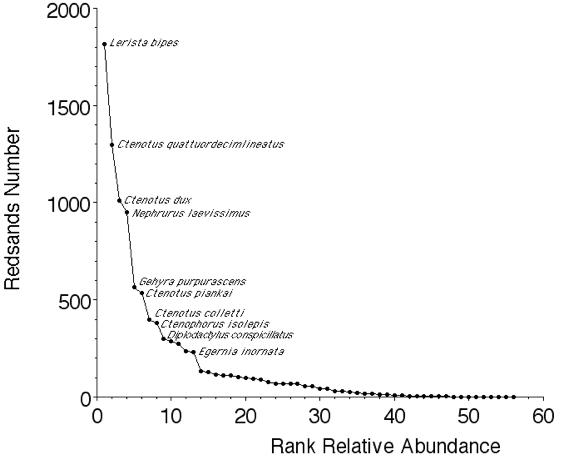
|
|
|
Species Accumulation Curves Species accumulation curves sometimes plot number of species versus number of individuals (Figure 3). Alternatively, they can plot number of species against sampling effort. Basically they are the inverse of relative abundance. Examples include Thompson et al. ( 2003; 2007) and Thompson and Withers (2003). Typically, number of species rises rapidly during early sampling but then levels off and approaches an asymptote, which can be used as an estimate of the total number of species present. Note, however, that rare species may continue to be captured long afterwards.
|
|
Species Diversity and Equitability Species density and relative importance have been combined in the concept of species diversity, sometimes also known as biodiversity, which increases with both increasing species richness and with increasing equality of importance among members of an assemblage, known as equitability (Tramer 1969). Species diversity is high when many species are rare or uncommon making it difficult to predict the identity of a species of a randomly chosen individual organism and low when an accurate prediction can be made. For example, lizard species densities from shrub-Acacia habitats in Australia are lower than those in sandy spinifex habitats (Pianka 1969, 1972). Many different indices of diversity have been proposed to quantify species diversity (Shannon 1948; Simpson 1949; Magurran 1988; Magurran and Henderson 2003; Magurran 2004). Species are weighted by abundance differently in each, but which index is used to quantify species diversity is somewhat arbitrary because all are highly correlated with each other. The simplest index is the inverse of Simpson's (1949) index of diversity [D = 1/ Σpi2] where pi is the proportion of individuals of species i. Other workers have used the more complex indices such as the Shannon-Weaver information theory index (Shannon 1948). Assumptions made by various indices differ, but are seldom discussed and are usually ignored. Many different questions can be asked about species richness and diversity such as "why do different communities contain different numbers of species with differing relative abundances, and how do such differences in species richness and importance affect other community properties such as trophic structure and community stability?" Diversity-Stability Controversy Long-held traditional ecological wisdom was that diversity led to stable ecological systems (Elton 1927; MacArthur 1955), but this idea was challenged on mathematical grounds by (May 1971, 1973). May constructed community matrices representing interactions between species in hypothetical model food webs and used matrix algebra to test them for mathematical stability. Using a random number generator, he varied number of species, connectance, and interaction strength. Stability decreased with increases in all three properties, directly opposite conventional ideas. May's methods and pivotal assumptions did not generate realistic model communities leading to ongoing debate over the relationship between diversity and stability (Lawlor 1978; Lane 1986; Haydon 1994; Tilman and Downing 1994; McCann 2000; Ives and Carpenter 2007). Rarity Understanding rarity constitutes a major challenge confronting ecologists (Main 1982; Pianka 2014). Main (1982) asked "Are rare species precious or dross? And, are they vital to community function?" Do rare species persist in more stable communities in spite of their rareness, or does the presence of rare species enhance the stability of ecosystems? Main (1982) suggested that one reason so many rare species exist may be that ecosystems have been "over-written many times after imperfect erasures" (incomplete extinctions). Consequently, current ecosystems contain numerous relicts of their predecessors assembled under different ecological conditions. Main suggested that rare species could be vital to long-term ecosystem sustainability, providing 'insurance' for the delivery of ecosystem functions by alternative means in the event of drastic environmental changes. Main's hypotheses could well be of considerable importance in these times of rapid climatic and ecological change (Thompson et al. 2003). Most species of Australian desert lizards are uncommon, making them difficult to study. Some are extremely rare to the point of vanishing rareness (Pianka 2011, 2014). Regardless of how rareness is defined, most ecologists concur that the majority of species are indeed uncommon (Gaston 1994; Kunin and Gaston 1997). Hanski (1982) and Magurran et al. (2003) distinguished between relatively abundant "core species" and uncommon "occasional species." Chronic rarity has proven to be exceedingly difficult to study, but, as mentioned above, rare species could well be quite important to community function (Main 1982; Morton and James 1988; Kunin and Gaston 1997; Pianka 2014). Apparent rarity can also be an artifact of an inadequate sampling method or unfavorable sampling period. For example, semifossorial snakes and skinks in tropical rainforests may appear very rare when using opportunistic survey methods (e. g. transect searches), but are really rather common when using pitfall trapping. Sampling Methods How, when and where animals are sampled as well as actual sample sizes themselves severely limit and impact estimates of diversity (Thompson et al. 2007). Common methods include timed visual encounter surveys, time-constrained searches (usually heavily biased by idiosyncrasies of observers), coverboards, pit trapping with drift fences, and trapping with funnel traps (Garden et al. 2002). Results and units of measure vary with methods employed. Although different species vary in trapability and detection probability, comparisons can be made between sites or over time intervals as long as the same methods are employed. Colwell (2013) provides a detailed discussion of statistical challenges encountered in sampling. Overlap and Similarity Often it is useful to compare species assemblages from different sites to assess how similar their faunas are. A wide variety of overlap and similarity indices are available (Horn 1966; Colwell and Futuyma 1971; Pianka 1973; Gotelli 2000). Which index is used is arbitrary. Saturation with Individuals and with Species In any closed ecosystem at equilibrium, all the energy of net production must be used up by consumers and decomposers for the system to have a balanced energy budget (Pianka 2000). Such an idealized system can be thought of as being saturated with individual organisms because all available energy is used and no more organisms could be supported. However, predators, by reducing densities of organisms at lower trophic levels, can prevent their prey populations from reaching otherwise maximal sustainable densities and thereby effectively preclude true saturation within that lower trophic level. If this is the case, only top predator populations reach a sort of "complete" saturation. Furthermore, anti-herbivore defenses of plants render much of the net primary productivity unusable by animal consumers and thus require that many plant tissues be routed directly through a community's decomposers. Communities, or portions thereof, can be kept from reaching saturation with individuals in other ways. Real ecological systems are almost never truly closed; rather, they usually both receive materials and energy from other systems and lose them to others. A community or a portion of a community that is not closed may be rarified by continual or sporadic removal of organisms. As a hypothetical example, consider a lake along a river. Both lake and river contain communities of phytoplankton and zooplankton, but the lake receives an inflow of river water containing no members of the lake community while losing water that contains some members of its community. Such a system can never become truly saturated because of the continual removal of organisms from it. Moreover, because the physical environment is changing continually and because it takes time to respond to these changes, populations and communities probably seldom reach equilibrium, although some very K-selected organisms may occasionally approach it (Pianka 1970). How much does the degree of saturation with individuals vary within and between communities? And how does the efficiency of energy transfer change with degree of saturation with individuals? Turnover rate of prey is usually highest at intermediate prey densities; moreover, such prey populations often support higher predator densities than larger prey populations. The immense practical value of such knowledge is readily apparent. Can communities be saturated with species? That is, is there a maximum number of different species that can exist within a given ecological system? If so, a new species introduced into such a community must either go extinct or cause the extinction of another species (which it then replaces). Conversely, successful invasion of a new species into a community without the extermination of an existing species would imply that the original community was not saturated with species (MacArthur 1965). Limited evidence suggests that portions of some communities may indeed be saturated with species, at least within habitats. R. H. MacArthur and colleagues demonstrated that bird species diversity is strongly correlated with foliage height diversity in a remarkably similar way on three continents: North America, South America, and Australia. Habitats with equal amounts of foliage (measured by leaf surface) in three layers (0 to 0.6, 0.6 to 8, and over 8 meters above ground) are richer in bird species than are habitats with unequal proportions of foliage in these three layers. The diversity of bird species is lowest in habitats with only one of these layers of vegetation, such as a grassland. Interestingly enough, knowledge of plant species diversity does not allow an improvement in the prediction of bird species diversity (MacArthur and MacArthur 1961), which suggests that birds recognize the structure rather than type of the vegetation. Despite the fact that avian niche space is partitioned in a fundamentally different way in Australia, bird species diversity within a given habitat on that continent is very close to what it is in a habitat with similar structure in North America (Recher 1969). In addition to illustrating that spatial heterogeneity regulates bird species diversity, the convergence of these data suggests that these avifaunas are saturated with species. However, bird species diversities were lower in a similar study in South America (Cody 1970). Also, such neat convergences in species densities of plants, insects, and desert lizards do not occur, which suggests that these groups may not always be saturated with species (Whittaker 1972; Pianka 1973). The number of species that can coexist at any point in space may have a distinct upper limit, as previously suggested; however, no obvious limitation exists on the number of species that can occur in a given area because horizontal replacement of species can allow coexistence of many more species than actually share the use of a common point in space within that area (MacArthur 1965). Indeed, the horizontal component of diversity ("between habitat" diversity) may be increasing continually during evolutionary time, whereas point diversities remain nearly constant (MacArthur 1965). Some upper limit on horizontal turnover of species also seems likely. Lizard species richness reaches its apex in the Australian deserts, where as many as 55 species co-occur in sympatry (Pianka 1969, 1986), In contrast, the richest sites in the Kalahari desert in southern Africa support only 22 species (Pianka 1971), whereas only a dozen species are found in North American deserts (Pianka 1967). Partitioning Diversity into Components Communities can differ in species diversity in several ways (MacArthur 1965, 1972; Pianka 2000). First, more diverse communities may contain a greater range of available resources (i.e., a larger total niche hypervolume space); second, their component species may, on average, have smaller niche breadths (i.e., each species might exploit a smaller fraction of the total niche hypervolume). The former corresponds roughly to "more niches" and the latter to "smaller niches." Third, two communities with identical niche space and mean niche breadth can still differ in species diversity if they differ in average degree of niche overlap because greater niche overlap means that more species can exploit any particular resource (this situation is described as greater resource sharing or "smaller exclusive niches"). Fourth, in communities that do not contain all the species they could conceivably support (i.e., those that are "unsaturated" with species), species diversity can vary with the extent to which all available resources are exploited by as many different species as possible (i.e., with the degree of saturation with species, or with the number of "empty niches"). Resources are seldom if ever wasted, even in communities that do not contain their full quota of species, because those species that do occur in such communities generally expand their activities and exploit nearly all the available resources, although their efficiency of exploitation may be less than that of some better-adapted species (most communities are probably effectively saturated with individuals even if they are not saturated with species.) Because thorough understanding of community species diversity inevitably requires analysis of resource partitioning among component populations, investigations of species diversity usually go hand in hand with the study of ecological niches. In practice, one is seldom able to study the species diversity of an entire community, and usually attention is focused on a portion of a community (an "assemblage") such as ants, birds, lizards, or trees. Using three major niche dimensions, total species diversity of an area can be partitioned into its spatial, temporal, and trophic components (Pianka 1973; Schoener 1974). Species replace one another along each of these niche dimensions, and diversity is generated by separation along each. Within-Habitat versus Between-Habitat Components of Diversity The spatial component of diversity is due to differential use of space by different populations; for convenience, it can be broken down into horizontal and vertical components. At a gross geographic level, species replace one another horizontally as one moves from one habitat to another (this is the between-habitat component of overall diversity). Similar replacement of species occurs both horizontally and vertically within habitats. For instance, like birds, arboreal lizards such as anoles often tend to partition a given habitat by occupying different vertical strata, such as low bushes, tree trunks, lower foliage, and high canopy (Williams 1983; Losos 2009). Ground-dwelling lizards generally partition microhabitats horizontally, with some using open spaces between shrubs and others exploiting the ground beneath or near specific types of vegetation such as grasses, shrubs, and trees (Pianka 1986). Different populations, by occupying different microhabitats, are thus able to avoid interspecific competition and coexist within a given habitat and contribute to within-habitat diversity. The within-habitat component of diversity is most easily distinguished from the between-habitat component in relatively homogeneous communities (heterogeneous communities such as edge communities and ecoclines include both components). However, even a homogeneous community has an internal structure in that it consists of a mosaic of repeatable horizontal and vertical patches. Because communities and habitats frequently blend into one another, it is sometimes difficult to distinguish between-habitat from within-habitat diversity. Where does one habitat "stop" and another "begin"? A sand ridge gradually gives way to a sand plain and the intertidal grades into the deeper benthic zone. The problem of defining a habitat can be overcome by the use of "point diversities," which consist of the species diversity occurring at a point in space (MacArthur 1965, 1972). Point diversities are difficult to estimate (one might have to wait a very long time to see all the species that use a particular point!). However, they should invariably be lower than any areal estimate of diversity because the different species in a community have each specialized somewhat as to the microhabitats they use. Reptiles captured in a given particular pitfall trap over a period of time may approximate point diversity. Informative maps of species density have been compiled and analyzed (Pianka 1967; Schall and Pianka 1978; Powney et al. 2010). For example, Shai Meiri at Tel-Aviv University compiled a global map of lizard species richness based on 4940 species demonstrating extremely high lizard species densities in Australia and Brazil (Meiri 2014). A map of species density for the species-rich skink genus Ctenotus in Australia is shown in Figure 5. 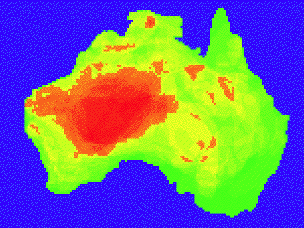
|
|
Provided that different resources are utilized, temporal separation, both daily and seasonally, of species' populations can allow coexistence of more species and hence may add to community diversity. Many instances of subtle differences in times of activity between populations are known, in addition to such conspicuous distinctions as those between nocturnal and diurnal animals. Yet another means by which community diversity may be enhanced is by trophic differences. Here again, in addition to the conspicuous differences between trophic levels (such as herbivores, omnivores, and carnivores), more subtle but nevertheless important differences exist between species even within a given trophic level in the prey they eat (Winemiller and Pianka 1990). Thus, different species of predators living in the same area tend to eat prey of different sizes and types, with the larger species taking larger prey items (this generalization applies to most fishes, lizards, carnivorous mammals, and hawks -- Pianka (2000). Moreover, the composition of the diet often varies markedly among potential competitors. Finally, diversity of plant defensive chemicals doubtlessly creates numerous different potential food niches for herbivores, especially insects the major food for lizards, thereby greatly facilitating trophic diversity at higher trophic levels. Diversity and Conservation Biology Unfortunately, many of the world's reptile species are now in peril or endangered due to inane anthropogenic activities such as overpopulation, global warming, fragmentation and usurpation of natural habitats (Botkin et al. 2007; Pianka 2012; Böhm et al. 2013). Estimates of species richness and diversity can be useful bio-indicators for environmental assessments in conservation biology (Faith 2008; Mott et al. 2010). Reptile species richness usually increases with structural habitat complexity (Pianka 1966, 1967; Fischer et al. 2004; Cartron et al. 2005; Samways 2005; Cayuela et al. 2006; Botkin et al. 2007). Critical habitat elements can sometimes been identified (Craig et al. 2011). Summary Species richness, also known as species density, is a simple count of how many species are present in a given area. Species diversity is more complex incorporating both number of species as well as their relative abundances; diversity increases with both species richness and the equitability of abundances. Diversity is high when many species are rare or uncommon making it difficult to predict the identity of a randomly chosen individual organism and as well as when species are more equal in abundance allowing a more accurate prediction to be made. Diversity can be partitioned into between-habitat and within-habitat components. In the limit, as space is made vanishingly small, point diversity represents all the species that use a particular point. Point diversities should invariably be lower than any areal estimate of diversity because the different species in a community have each specialized somewhat as to microhabitats used. Resource partitioning among sympatric species contributes to diversity in several ways: narrower niches and greater tolerable niche overlap allow more species to coexist on a given resource base without competitive exclusion. Sampling methods and sample size limit and constrain estimates of diversity. Which one of many different indices is used to quantify species diversity in any given study is arbitrary because all are highly correlated. Estimates of species richness and diversity are useful bio-indicators for environmental assessments in conservation biology. Web Resources EcoSim (Gotelli and Entsminger 2003), EstimateS (Colwell 2013). References Böhm, M., et al. (2013). The conservation status of the world's reptiles. Biological Conservation, 157, 372-385. Download pdf Botkin, D. B., H. Saxe, M. B. Araújo, R. Betts, R. H. W. Bradshaw, T. Cedhagen, P. Chesson, T. P. Dawson, J. R. Etterson, D. P. Faith, S. Ferrier, A. Guisan, C. Margules, Loehle C, M. New, M. J. Sobel and D. R. B. Stockwell (2007). Forecasting the Effects of Global Warming on Biodiversity. Bioscience, 57(3), 227-236. Cartron, J. E., R. Ceballos and R. S. Felger (2005). Biodiversity, Ecosystems, and Conservation in Northern Mexico. Oxford University Press. Cayuela, L., D. J. Golicher, J. M. R. Benayas, M. Gonzalez-Espinosa and N. Ramirez-Marcial (2006). Fragmentation, disturbance and tree diversity conservation in tropical montane forest. Journal of Applied Ecology, 43, 1172-1181. Cody, M. L. (1970). Chilean bird distribution. Ecology, 51, 455-463. Colwell, R. K. (2013). EstimateS: Statistical estimation of species richness and shared species from samples. Version 9. User's Guide and application published on line at: http://purl.oclc.org/estimates. Colwell, R. K., A. Chao, N. J. Gotelli, S. Y. Lin, C. X. Mao, R. L. Chazdon and J. T. Longino (2012). Colwell, R. K., A. Chao, N. J. Gotelli, S.-Y. Lin, C. X. Mao, R. L. Chazdon, and J. T. Longino. 2012. Models and estimators linking individual-based and sample-based rarefaction, extrapolation, and comparison of assemblages. Journal of Plant Ecology, 5, 3-21. Colwell, R. K. and D. J. Futuyma (1971). On the measurement of niche breadth and overlap. Ecology, 52, 567-576. Craig, M. D., A. M. Benkovic, A. H. Grigg, E. S. J. Giles, A. H. Hardy, P. A. Fleming and R. J. Hobbs (2011). How many mature microhabitats does a slow-recolonising reptile require? Implications for restoration of bauxite minesites in south-western Australia. Australian Journal of Zoology, 59(1), 9-17. Elton, C. S. (1927). Animal Ecology. Sidgwick and Jackson, London. Faith, D. P. (2008). Threatened Species and the Potential Loss of Phylogenetic Diversity: Conservation Scenarios Based on Estimated Extinction Probabilities and Phylogenetic Risk Analysis. Conservation Biology, 22(6), 1461-1470. Fischer, J., D. B. Lindenmayer and A. Cowling (2004). The challenge of managing multiple species at multiple scales: reptiles in an Australian grazing landscape. Journal of Applied Ecology, 41, 32-44. Garden, J., C. A. McAlpine, H. P. Possingham and D. N. Jones (2002). Using multiple survey methods to detect terrestrial reptiles and mammals: what are the most successful and cost-efficient combinations? Wildlife Research, 34, 218-227. Gaston, K. J. (1994). Rarity. Chapman and Hall. Gotelli, N. J. (2000). Null model analysis of species co-occurrence patterns. Ecology, 81(9), 2606-2621. Gotelli, N. J. and R. K. Colwell (2001). Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters, 4, 379-391. Gotelli, N. J. and G. L. Entsminger (2003). EcoSim: Null models software for ecology. Acquired Intelligence Inc. & Kesey-Bear. Burlington, VT. 05465, on line at http://homepages.together.net/~gentsmin/ecosim.htm Hanski, I. (1982). Dynamics of Regional Distribution: The Core and Satellite Species Hypothesis. Oikos, 38, 210-221. Haydon, D. T. (1994). Pivotal Assumptions Determining the Relationship between Stability and Complexity: An Analytical Synthesis of the Stability-Complexity Debate. The American Naturalist, 144, 14-29. Hill, M. O. (1973). Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 54: 427-432. Horn, H. S. (1966). Measurement of overlap in comparative ecological studies. American Naturalist, 100, 419-424. Inger, R. F. and R. K. Colwell (1977). Organization of contiguous communities of amphibians and reptiles in Thailand. Ecological Monographs, 47, 229-253. Ives, A. R. and S. R. Carpenter (2007). Stability and Diversity of Ecosystems. Science, 317, 58-62. Kunin, W. E. and K. J. Gaston, Eds. (1997). The biology of rarity: causes and consequences of rare-common differences. Chapman and Hall. Lane, P. A. (1986). Symmetry, change, perturbation, and observing mode in natural communities. Ecology, 67, 223-239. Lawlor, L. R. (1978). A comment on randomly constructed model ecosystems. The American Naturalist, 112, 445-447. Losos, J. B. (2009). Ecology and Adaptive Radiation of Anoles. University of California Press, Berkeley, CA. MacArthur, R. H. (1955). Fluctuations of animal populations, and a measure of community stability. Ecology, 36, 533-536. MacArthur, R. H. (1960). On the relative abundance of species. American Naturalist, 94, 25-36. MacArthur, R. H. (1965). Patterns of species diversity. Biological Reviews, 40, 510-533. MacArthur, R. H. (1972). Geographical Ecology. Harper and Row, New York. MacArthur, R. H. and J. W. MacArthur (1961). On bird species diversity. Ecology, 42, 594-598. Magurran, A. (1988). Ecological Diversity and Its Measurement. . Princeton Univ. Press, Princeton, New Jersey, USA. Magurran, A. (2004). Measuring Biological Diversity. Blackwell, Oxford. Magurran, A. E. and P. Henderson (2003). Explaining the excess of rare species in natural species abundance distributions. Nature, 422(6933), 714-716. Main, A. (1982). Rare species: precious or dross? Australian Academy of Science, Canberra. May, R. M. (1971). Stability in multispecies community models. Mathematical Biosciences, 12, 59-79. May, R. M. (1973). Stability and complexity in model ecosystems. Princeton University Press, Princeton. McCann, K. S. (2000). The diversity-stability debate. Nature, 405, 228-233. Meiri, S. (2014). "Shai Meiri's Lab." 2014. http://shaimeirilab.weebly.com/research-interests.html Morton, S. R. and C. D. James (1988). The diversity and abundance of lizards in arid Australia: a new hypothesis. The American Naturalist, 132(2), 237-256. Motomura, I. (1932). A statistical treatment of associations. Japanese Journal of Zoology, 44, 379-383. Mott, B., R. A. Alford and L. Schwarzkopf (2010). Tropical reptiles in pine forests: Assemblage responses to plantations and plantation management by burning. Forest Ecology and Management, 259(5), 916-925. Pianka, E. R. (1966a). Convexity desert lizards and spatial heterogeneity. Ecology, 47(6), 1055-59. Pianka, E. R. (1966b). Latitudinal gradients in species diversity: A review of concepts. American Naturalist 100: 33-46. Celebrated in Asilomar in 2016. Recognized as a "Historical Comment" in 2017: Latitudinal Gradients in Species Diversity: Reflections on Pianka's 1966 Article and a Look Forward. Pianka, E. R. (1967). On Lizard Species Diversity - North American Flatland Deserts. Ecology, 48(3), 333-351. Pianka, E. R. (1969). Habitat specificity, speciation, and species density in Australian desert lizards. Ecology, 50, 498-502. Pianka, E. R. (1970). On r and K selection. American Naturalist, 104(940), 592-597. Pianka, E. R. (1971). Lizard species density in the Kalahari desert. Ecology, 52, 1024-1029. Pianka, E. R. (1972). Zoogeography and speciation of Australian desert lizards: An ecological perspective. Copeia, 1972, 127-145. Pianka, E. R. (1973). The structure of lizard communities. Annual Review of Ecology and Systematics, 4, 53-74. Pianka, E. R. (1977). Reptilian species diversity. Biology of the Reptilia. In C. Gans and D. W. Tinkle (ed). Academic Press. New York: 1-34. Pianka, E. R. (1986). Ecology and Natural History of Desert Lizards: Analyses of the Ecological Niche and Community Structure. Princeton Univ. Press, Princeton, New Jersey. Pianka, E. R. (2000). Evolutionary Ecology. Addison Wesley Longman, San Francisco, CA. Pianka, E. R. (2011). Notes on the ecology of some uncommon skinks in the Great Victoria Desert. Western Australian Naturalist, 28, 50-60. Western Australian Naturalist, 28, 50-60. Pianka, E. R. (2012). Can humans share Spaceship Earth? Amphibian and Reptile Conservation, 6, 1-24. Amphibian and Reptile Conservation, 6, 1-24. Pianka, E. R. (2014). Rarity in Australian desert lizards. Austral Ecology, 39, 214-224. Powney, G. D., R. Grenyer, C. D. L. Orme, I. P. E. Owens and S. Meiri (2010). Hot, dry and different: Australian lizard richness is unlike that of mammals, amphibians and birds. Global Ecology and Biogeography, 19, 386-396. Powney-etal-2010.pdf Recher, H. (1969). Bird species diversity and habitat diversity in Australia and North America. American Naturalist, 103, 75-80. Samways, M. J. (2005). Insect Diversity Conservation. Cambridge University Press. Schall, J. J. and E. R. Pianka (1978). Geographical trends in numbers of species. Science, 201, 679-686. Schoener, T. W. (1974). Resource partitioning in ecological communities. Science, 185(4145), 27-39. Shannon, C. (1948). The mathematical theory of communication. University of Illinois Press, Urbana. Simpson, E. H. (1949). Measurement of diversity. Nature, 163, 688. Thompson, G. G., S. A. Thompson, P. Withers and J. Fraser (2007). Determining adequate trapping effort and species richness using species accumulation curves for environmental impact assessments. Austral Ecology, 32, 570-580. Thompson, G. G. and P. C. Withers (2003). Effect of species richness and relative abundance on the shape of the species accumulation curve. Austral Ecology, 28, 355-360. Austral Ecology, 28, 355-360 Thompson, G. G., S. A. Thompson, P. C. Withers and E. R. Pianka. (2003). Diversity and abundance of pit-trapped reptiles of arid and mesic habitats in Australia: Biodiversity for Environmental Impact Assessments. Pacific Conservation Biology 9: 120-135. Thompson, G. G., P. C. Withers, E. R. Pianka and S. A. Thompson (2003). Assessing biodiversity with species accumulation curves; inventories of small reptiles by pit-trapping in Western Australia. Austral Ecology, 28(4), 361-383. Tilman, D. (1996). Biodiversity: Population Versus Ecosystem Stability. Ecology 77: 350-363. Tilman, D. (1999). The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80: 1455-1474. Tilman, D. and J. A. Downing (1994). Biodiversity and stability ingrasslands. Nature 367: 363-365. Tramer, E. (1969). Bird species diversity: components of Shannon's formula. Ecology, 50, 927-929. Whittaker, R. (1972). Evolution and measurement of species diversity. Taxon, 21, 213-251. Whittaker, R. (1975). Communities and Ecosystems. MacMillan, New York. Williams, E. E. (1983). Ecomorphs, Faunas, Island Size, and Diverse End Points in Island Radiations of Anolis. Lizard Ecology: Studies of a Model Organism. In R. B. Huey, E. R. Pianka and A. Schoener (ed). Harvard University Press. Cambridge, Massachusetts. Winemiller, K. O. and E. R. Pianka (1990). Organization in natural assemblages of desert lizards and tropical fishes. Ecological Monographs, 60(1), 27-55. Ecological Monographs 60: 27-55. Last updated 25 March 2015 by Eric R. Pianka |