
 The largest species of Australian lizard, the perentie Varanus giganteus. To learn more about this lizard, click here Eric R. Pianka Department of Zoology, University of Texas, Austin, Texas 78712-1064 KEYWORDS: Evolution, body size, comparative methods, independent contrasts, monitor lizards, phylogenetic systematics, varanid lizards Abstract. Modern comparative methods allow examination of the probable course of evolution in a lineage of lizards (family Varanidae, genus Varanus). Within this genus, body mass varies by nearly a full five orders of magnitude. The fossil record and present geographic distribution suggest that varanids arose over 65 million years ago in Laurasia and subsequently dispersed to Africa and Australia. Two major lineages have undergone extensive adaptive radiations within Australia: one evolved dwarfism (subgenus Odatria = pygmy monitors), whereas the other Australian lineage (subgenus Varanus) remained large and several of its members evolved gigantism. Body sizes of extant varanid species are plotted on a phylogeny and probable sizes at ancestral nodes are inferred from those of their descendents. Felsenstein phylogenetically independent contrasts, coupled with information on branch lengths, are exploited to identify several likely instances of relatively rapid evolution of body size, both between and within clades. Numerous questions about the evolution of size in this genus within a historical/geographical perspective remain to be answered. A century ago, Edward Drinker Cope (1896) noted that body size frequently increased during the evolutionary history of a phylogenetic lineage, giving rise to "Cope's law of phyletic size increase." More recently, Schmidt-Nielsen (1984), in his title "Scaling. Why is animal size so important?", discussed general problems of size and scale, allometry, constraints imposed by body plans, plus the effects of size on a host of physiological processes and animal performances such as locomotion. Calder (1984) stressed how life historical traits are constrained by and covary with body size. For example, generation time is strongly positively correlated with size, whereas rates of increase covary negatively with size. Phylogenetic systematics has revitalized comparative evolutionary biology because this powerful approach allows analyses of the probable course of evolution (Ridley 1983; Felsenstein 1985; McLennan et al. 1988; Brooks and McLennan 1991; Harvey and Pagel 1991, Garland et al. 1991; Garland 1992). In a now classic paper, appropriate statistical procedures useful in factoring out phylogenetic effects and estimating ancestral states and rates of evolution were first worked out by Felsenstein (1985). These methods have been extended by Felsenstein (1988), Grafen (1989), Maddison (1990), Harvey and Pagel (1991), Harvey and Purvis (1991), Martins and Garland (1991), Garland (1992), Garland et al. (1992), and others. Here I apply some of these potent comparative techniques to trace the probable course of evolution of body size within a lineage of lizards. Possible scenarios for evolution of size in this lizard lineage are discussed and many questions worthy of further research are raised. Evolution and Diversity of the Varanidae Varanus are morphologically conservative, but vary in mass by nearly five orders of magnitude. To my knowledge, no other terrestrial animal genus exhibits such a range of size variation. There is proportionately almost as much difference in mass among species within the genus Varanus as there is between an elephant and a mouse [Footnote: Mammals vary in size from 3 gm shrews to whales that weigh over 50 tons, but constitute an entire subclass. No mammal genus exhibits size variation anywhere near as extensive as Varanus. Varanids are sufficiently conservative morphologically that taxonomic ranking at the generic level seems justified. Even if the genus Varanus is eventually broken up into several closely-related genera, the comparison with size variation in mammals is still striking.] These properties, coupled with the fact that a phylogeny has recently become available, make varanid lizards a nearly ideal model system for examining patterns of size evolution. 
Catching a Varanus tristis at Red Sands. All living members of the Varanidae, commonly known as monitor lizards, are placed in a single genus Varanus, generally considered to be monophyletic (Baverstock et al. 1993; Green and King 1993; King et al. 1991; Pregill et al. 1986). Many monitor species are large and impressive ö they are often the centerpiece of reptile house exhibits. Varanus, which are not particularly tractable research subjects, have also received an extraordinary amount of attention from dedicated students. All but one species, the frugivorous Varanus olivaceus of the Philippines (Auffenberg 1988), are active predators and eat quite large prey relative to their own body size (Pianka 1982, 1994; but see Losos and Greene 1988 and Auffenberg 1994 for dissenting views). Many monitor lizards are top predators (Losos and Greene 1988; Pianka 1994). Some species are aquatic, others terrestrial, while still others are saxicolous and/or semi-arboreal or truly arboreal. Monitor lizards live in a wide variety of habitats, ranging from mangrove swamps to dense forests to savannas to arid deserts. McDowell and Bogert (1954) recognized the lizard superfamily Varanoidea which consists of the lineage of lizards that gave rise to the family Varanidae plus several other lineages including North American Helodermatidae (Gila monsters), Lanthanotidae from Borneo, as well as some extinct taxa such as gigantic marine mosasaurs and the upper Cretaceous Mongolian Estesia known only from fossils (Norell et al. 1992). Pregill et al. (1986) subsequently restricted Varanoidea to Varanidae (Varaninae plus Lanthanotinae) and Helodermatidae. The earliest known varanoid fossils date from the upper Cretaceous, Paleocene, and Eocene of North America, Europe, and Mongolia (McDowell and Bogert 1954; Norell et al. 1992), suggesting an ancient Laurasian origin for the group. Many of these fossils are very similar to present-day varanids, an indication of the conservativeness and staying power of this basic body plan. Most students of Varanus believe that the genus arose in Eurasia where the majority of Merten's subgenera still occur today (Baverstock et al. 1993; Greer 1989; King and Green 1993). Varanids are usually considered to be a late Tertiary invader of Australia because the earliest Australian fossils are from the middle Miocene of South Australia. Others (Hutchinson and Donnellan 1993; K. Aplin, pers. comm.) have, however, argued for a Gondwanan origin for varanids, which remains a definite possibility.  ERP implants a transmitter in male E Varanus tristis The sole surviving varanoids in the New World are the Gila monster, Heloderma suspectum, and its Mexican congener, the Beaded lizard, Heloderma horridus (Bogert and del Campo 1956). By nearctic-neotropical standards, Heloderma are large lizards (0.35-0.45 m SVL ) ö yet, when compared to an average varanid they are only moderate-sized lizards. Currently, 44 extant species of Varanus are recognized worldwide, some 27 of which occur in Australia. On the basis of morphological evidence, Mertens (1942, 1958, 1963) erected the first taxonomy. He recognized ten subgenera (Table 1), seven of which contain only a single species. Two speciose subgenera, Odatria and Varanus, have undergone adaptive radiations in Australia. Almost all 17 members of Odatria are small and all are restricted to Australia [Footnote: Mertens misplaced prasinus in Odatria -- it and its close relatives belong to an Asian clade (see below).], suggesting that this lineage of pygmy monitors arose and diversified within Australia. Most members of the subgenus Varanus are also confined to Australia. Only four varanid species occur in all of Africa (albigularis, exanthematicus, griseus and niloticus), and these were assigned to three different subgenera by Mertens. Fourteen other species (belonging to eight of Mertens' ten subgenera) are variously distributed across Asia and southeast Asia (bengalensis, dumerili, flavescens, indicus [also found in northern Australia], jobiensis (formerly karlschmidti Bohme 1991), komodoensis, olivaceus, prasinus [also found in northern Australia] and its close relatives (beccari, bogerti, telenesetes), rudicollis, salvadori and salvator). One other little-known species (yemenensis) is restricted to Arabia. The highest species densities of Varanus occur in Australia, where as many as six species occur together in sympatry in the arid desert interior (Pianka 1994) and up to ten species coexist in Arnhem Land in wet northern tropical Australia (Cogger and Heatwole 1981; S. Sweet, pers. comm.).  Two distinct evolutionary lineages have undergone extensive adaptive radiations in Australia: the pygmy monitor subgenus Odatria, and the subgenus Varanus, which includes the larger species of Australian monitors plus several species found nearby outside Australia
(indicus, prasinus and teriae appear to have recently reached Australia from New Guinea).
Two distinct evolutionary lineages have undergone extensive adaptive radiations in Australia: the pygmy monitor subgenus Odatria, and the subgenus Varanus, which includes the larger species of Australian monitors plus several species found nearby outside Australia
(indicus, prasinus and teriae appear to have recently reached Australia from New Guinea).
World's smallest monitor lizard, a neonate Varanus brevicauda, approximately actual size, (SVL = 52 mm, weight 1.4 gms). --------------------> Australian monitors range in size from the diminutive pygmy monitor Varanus brevicauda (0.2 m in total length, 8-10 gms) to the largest living lizard in Australia, the perentie, Varanus giganteus (total length may exceed 2.0 m, mass 17 kg). The typical monitor lizard threat posture and behavior has been conserved in the evolution of tiny monitors such as brevicauda, which hiss and lunge with throat inflated as if they are a serious threat. Even more awesome are Komodo monitors (Varanus komodoensis) of the Lesser Sunda Islands of Indonesia, which attain total lengths of over 3 m and weights of more than 150 kg (Auffenberg 1981). Komodo monitors, however, are themselves dwarfed by the largest known terrestrial lizard, a closely-related gigantic varanid formerly assigned to the genus Varanus. Megalania prisca is a Pleistocene fossil (19,000-26,000 years BP) from Australia, estimated to have reached 7 m in total length and to have weighed more than 600 kg (Hecht 1975; Auffenberg 1981; Rich 1985). [Cladistic systematics will undoubtedly require that the fossil taxon Megalania eventually be reassigned to the genus and subgenus Varanus (Kluge, pers. comm.)] These spectacular creatures must have been even more formidable than modern-day saltwater crocodiles. The major prey of these gigantic monitor lizards is thought to have been large diprotodont marsupials (rhinoceros-sized beasts, now extinct, that were relatives of wombats and koalas). Being contemporaneous with aboriginal humans in Australia, in all probability, Megalania also ate Homo sapiens. Megalania teeth were over 2 cm long, curved, with the rear edge serrated for cutting and tearing the skin and flesh of its prey as these powerful predators pulled back on their bite. Many other species of Varanus also possess such teeth. Several authors have suggested that Varanus komodoensis and Megalania prisca are/were ecological equivalents of large saber-toothed cats, using a slashing bite to disembowl large mammals (Akersten 1985; Auffenberg 1981; Losos and Greene 1988). Water buffalo as large as 590 kg have been killed by Varanus komodoensis, more than three times their own mass (Auffenberg 1981). [Cladistic systematics will undoubtedly require that the fossil taxon Megalania eventually be reassigned to the genus and subgenus Varanus (Kluge, pers. comm.)] Phylogeny of Varanids In addition to Mertens (1942, 1958, 1963), several other efforts have been made to place extant varanids into groups. King and King (1975) and King (1990) studied karyotypes of 27 varanid species and identified six different independent karyomorph groups defined on the basis of chromosome markers such as fixed multiple pericentric inversions and the presence of acrocentric or metacentric micro-chromosomes. Clusters of species were placed into each of these six karyomorph groups. Other workers have also attempted to place various species of Varanus into groups based on hemipeneal morphology (Branch 1982; Bohme 1988), and lung anatomy (Becker et al. 1989). Phylogenetically informative data from chromosomal, hemipeneal and lung morphology are largely (although not entirely) concordant with microcomplement fixation (MCF) data reported below on which this paper is based. Using microcomplement fixation (MCF), King et al. (1991) and Baverstock et al. (1993) examined relationships of 30 of the 44 extant species of Varanus. Reciprocal testing produced immunological distances and generated a phylogeny for 14 taxa (Figure 1). Although less reliable, one-way tests added another 18 taxa to the tree, two of which are subspecies (Figure 2). Despite the methodological weakness of one-way tests, the striking concordance with biogeography (Figure 2) lends some credance to this phylogeny. Seven of the 14 missing taxa are small Australian monitor species generally considered to belong in the subgenus Odatria. Three other missing taxa are the Philippine olivaceus, the African exanthematicus [closely allied to albigularis (Mertens 1942, 1958), which is included], plus the Arabian yemenensis [closely-related to albigularis (Bohme 1988, 1991)]. The remaining four missing species are newly recognized close relatives of prasinus (Sprackland 1991a), which is included. For obvious reasons, the fossil M. prisca is also missing from Figure 2. Unfortunately, this tree could not be rooted (one outgroup, Lanthanotus, is rare and unavailable, whereas the 
The sister group to Varanus is the earless monitor from Borneo. other, Heloderma, was too distantly related to cross react to Varanus antiserum). Because the tree is unrooted, the apparent basal tetrachotomy may not in fact be basal. Note, however, that to root this tree so that Australian species could be represented as monophyletic requires either 1) an Asian root or an African root with an exceedingly unlikely trans-oceanic crossing between Africa and Australia, or 2) rooting the tree at the base of or within either of the Australian groups, which in turn would require highly unlikely separate invasions of Asia and Africa from Australia. The unlikelihood of such trans-oceanic movements between Australia and Africa, in either direction, coupled with present day geographical distributions, justify as a working hypothesis the recognition of four clades: the 1) Australian Odatria pygmy monitor clade, 2) an African clade, 3) an Asian clade, and 4) the Australian clade of large monitors (includes komodoensis and salvadori). (The fifth "lineage" leading to eremius is only weakly supported as it relies on one-way tests and is not supported by non-MCF data [The small size of eremius, its geographical location in Australia, morphological similarity to other Odatria, and hemipeneal anatomy all suggest that eremius will ultimately be found to belong in the subgenus Odatria.]). Obviously, any of these putative "clades" would be destroyed if the tree is rooted within one of them. However, because these putative clades do make biogeographical sense, I accept them tentatively. 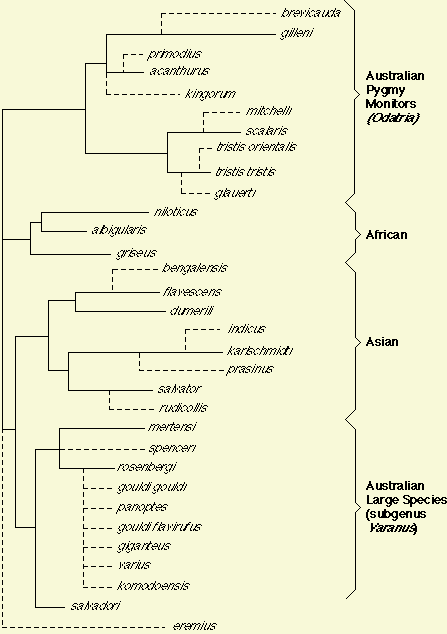
We are collecting DNA sequence data to infer phylogeny for a phylogenetic study of evolution in monitor lizards of the genus Varanus. Two of many such traits that we will plot on a phylogeny are body size and clutch size (for an example, see Pianka 1995 Amer. Natur. 146: 398-414). To read about Varanus in the Great Victoria desert of Western Australia, click Desert Varanus. To go to Graham Thompson's list of Varanus references, click Varanus References. |