Optimal Reproductive Tactics
Eric R. Pianka
Natural selection recognizes only one currency: successful offspring. Yet even though all living organisms have presumably been selected to maximize their own lifetime reproductive success, they vary greatly in exact modes of reproduction. Some, such as most annual plants, a multitude of insects, and certain fish like the Pacific salmon, reproduce only once during their entire lifetime. These “big-bang” or semelparous reproducers typically exert a tremendous effort in this one and only opportunity to reproduce -- in fact their exceedingly high investment in reproduction may contribute substantially to their own demise.
Many other organisms, including perennial plants and most vertebrates, do not engage in such suicidal bouts of reproduction but reproduce again and again during their lifetime. Such organisms are iteroparous (repeated parenthood). Even within organisms that use either the big-bang or the iteroparous tactic, individuals and species differ greatly in numbers of progeny produced. Annual seed set of different species of trees ranges from a few hundred or a few thousand in many oaks (which produce relatively large seeds -- acorns) to literally millions in redwood trees. Seed production may vary greatly even among individual plants of the same species grown under different environmental conditions; an individual poppy produces as few as four seeds under stress conditions, but as many as a third of a million seeds when grown under conditions of high fertility. Fecundity is equally variable among fish; the large ocean sunfish, Mola mola, is perhaps the most fecund of all vertebrates with a clutch of 200 million tiny eggs.

Ocean Sunfish, Mola mola
A female codfish also lays millions of relatively tiny eggs. Most elasmobranchs (sharks, skates, and rays), however, produce considerably fewer but much larger offspring. Variability of clutch and/or litter size is not nearly so great among other classes of vertebrates, but it is still significant. Among lizards, for example, clutch size varies from a fixed clutch of one in some geckos and Anolis to as many as 40 in certain horned lizards (Phrynosoma) and large Ctenosaurus and Iguana. Timing of reproduction also varies considerably among organisms.
Due to the finite chance of death, earlier reproduction is always advantageous, all else being equal. Nevertheless, many organisms postpone reproduction. The century plant, an Agave, devotes years to vegetative growth before suddenly sending up its inflorescence (some related monocots bloom much sooner). Delayed reproduction also occurs in most perennial plants, many fish such as salmon, a few insects like cicadas, some lizards, and many mammals and birds, especially among large seabirds.
High fecundity early in life is often correlated with decreased fertility later on, an excellent example of the principle of allocation. When early fecundity is lower, plots of fecundity versus age are flatter. Innumerable other examples of the diversity of existing reproductive tactics could be listed. Clearly, natural selection has shaped observed reproductive tactics, with each presumably corresponding in some way to a local optimum that maximizes an individual’s lifetime reproductive success in its particular environment.

In different strains of white Leghorn domestic chickens, fecundity drops off faster with age in birds that lay many eggs early in life, as might be anticipated from the principle of allocation.
Population biologists would like to understand factors that influence evolution of various modes of reproduction.
Reproductive Effort
How much should an organism invest in any given act of reproduction?
R. A. Fisher (1930) anticipated this question long ago:
“It would be instructive to know not only by what physiological mechanism a just apportionment is made between the nutriment devoted to the gonads and that devoted to the rest of the parental organism, but also what circumstances in the life history and environment would render profitable the diversion of a greater or lesser share of the available resources towards reproduction.”
Fisher clearly distinguished between the proximate factor (physiological mechanism) and the ultimate factors (circumstances in the life history and environment) that determine the allocation of resources into reproductive versus non-reproductive tissues and activities. Reproductive effort, an organism’s investment in any current act of reproduction, has played a central role in thinking about reproductive tactics. Although reproductive effort is conceptually quite useful, it is difficult to quantify adequately. Ideally, an operational measure of reproductive effort would include direct material and energetic costs of reproduction as well as risks associated with a given level of current reproduction.
Temporal patterns of collection and expenditure of matter and energy are also important. Many organisms gather and store materials and energy during time periods that are unfavorable for successful reproduction but then expend these same resources on reproduction during a later, more suitable time. The large first clutch of a fat female lizard that has just over wintered may actually represent a smaller investment in reproduction than her subsequent smaller clutches that must be produced with considerably diminished energy reserves. Reproductive effort could perhaps be best measured operationally in terms of the effects of various current levels of reproduction upon future reproductive success.
Instantaneous ratios of reproductive tissues over total body tissue are sometimes used as a crude first approximation of an organism’s reproductive effort (both weights and calories have been used). The proportion of total resources available to an organism allocated to reproduction varies widely among organisms. Among different species of plants, energy expenditure on reproduction, integrated over a plant’s lifetime, ranges from near zero to as much as 40 percent. Annual plants tend to expend more energy on reproduction than most perennials (about 14 to 30 percent versus 1 to 24 percent). An experimental study of the annual euphorb Chamaesyce hirta showed that calories allocated to reproduction varied directly with nutrient availability and inversely with plant density and competition.
Optimal reproductive effort can be examined from the perspective of the dichotomy for the apportionment of energy into reproductive versus non-reproductive (somatic) tissues, organs, and activities. Somatic tissues are clearly necessary for acquisition of matter and energy; at the same time, an organism’s soma is of no selective value except inasmuch as it contributes to that organism’s lifelong production of successful offspring. Allocation of time, energy, and materials to reproduction in itself usually decreases growth of somatic tissues and often reduces future fecundity.
Increased reproductive effort may also cost by reducing survivorship; this is easily seen in the extreme case of big-bang reproduction, in which an organism puts everything available into one suicidal bout of reproduction and then dies. More subtle changes in survivorship also occur with minor alterations in reproductive effort.
How great a risk should an optimal organism take with its soma in any given act of reproduction? This question can be explored using the concept of residual reproductive value, which is simply age-specific expectation of all future offspring beyond those immediately at stake. To maximize its overall lifetime contribution to future generations, an optimal organism should weigh profits of its immediate prospects of reproductive success against costs to its long-term future prospects. An individual with a high probability of future reproductive success should be more hesitant to risk its soma in present reproductive activities than another individual with a lower probability of reproducing successfully in the future. Moreover, to the extent that present reproduction decreases expectation of further life, it may reduce residual reproductive value directly. For both reasons, current investment in reproduction should vary inversely with expectation of future offspring.
In the rotifer Asplanchna, probability of survival and expectation of future offspring both decrease with increased age-specific fecundity.
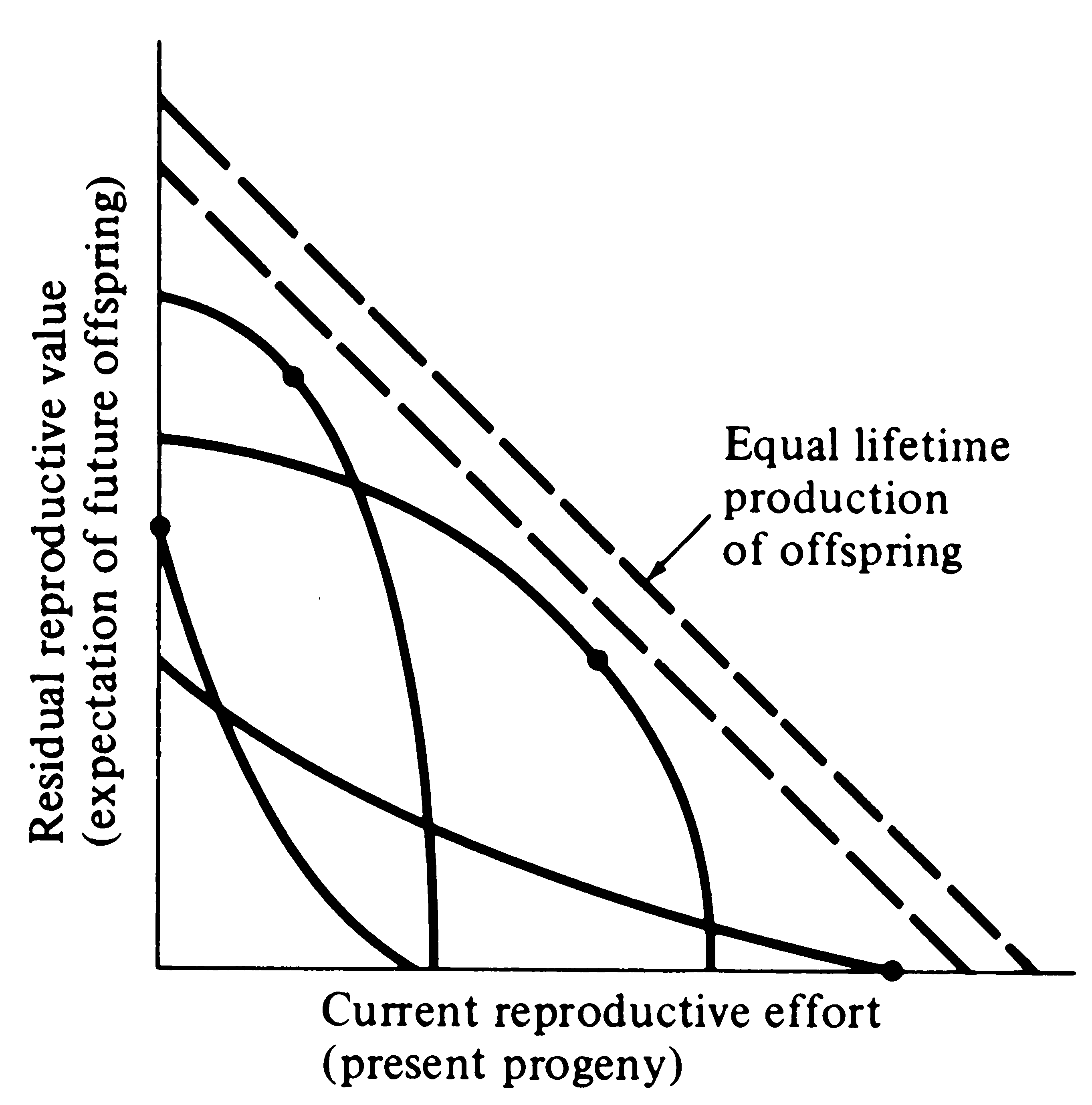 Figure. Trade-offs between current reproductive effort and expectation of future offspring at any particular instant (or age). Four curves relate costs in future progeny to profits in present offspring (and vice versa), with a dot marking the reproductive tactic that maximizes total possible lifetime reproductive success. Concave upward curves lead to all-or-none “big-bang” reproduction, whereas convex upward curves result in repeated reproduction (iteroparity). (From Pianka 1976).
Figure. Trade-offs between current reproductive effort and expectation of future offspring at any particular instant (or age). Four curves relate costs in future progeny to profits in present offspring (and vice versa), with a dot marking the reproductive tactic that maximizes total possible lifetime reproductive success. Concave upward curves lead to all-or-none “big-bang” reproduction, whereas convex upward curves result in repeated reproduction (iteroparity). (From Pianka 1976).
Several possible different forms for the inverse interaction between reproductive effort and residual reproductive value are depicted in the above figure. Curves in this simple graphical model relate costs and profits in future offspring, respectively, to profits and costs associated with various levels of current reproduction, the latter measured in present progeny. Each curve depicts all possible tactics available to a given organism at a particular instant, ranging from a current reproductive effort of zero to all-out big-bang reproduction. In a stable population, immediate progeny and offspring in the more distant future are of equivalent value in perpetuation of an organism’s genes; here a straight line with a slope of minus 45° represents equal lifetime production of offspring. A family of such lines (dashed) is plotted in the figure.
An optimal reproductive tactic exists at the point of intersection of any given curve of possible tactics with the line of equivalent lifetime reproductive success that is farthest from the origin; this level of current reproduction maximizes both reproductive value at that age and total lifetime production of offspring (dots in Figure). For any given curve of possible tactics, all other tactics yield lower returns in lifetime reproductive success. The precise form of the trade-off between present progeny and expectation of future offspring thus determines the optimal current level of reproductive effort at any given time. Note that concave-upward curves always lead to big-bang reproduction, whereas convex-upward curves result in iteroparity because reproductive value and lifetime reproductive success are maximized at an intermediate current level of reproduction.
In many organisms, residual reproductive value first rises and then falls with age; optimal current level of reproduction rises as expectation of future offspring declines. Current fecundity also increases as residual reproductive value falls, but the surface for a big-bang reproducer is always concave upward. Exact shapes of these tradeoff surfaces depend on the actual reproductive tactic taken by an organism as well as on immediate environmental conditions for foraging, reproduction, and survival. The precise form of trade-offs between present progeny and expectation of future offspring is influenced by numerous factors, including predator abundance, resource availability, and numerous aspects of the physical environment. Unfavorable conditions for immediate reproduction decrease costs of allocating resources to somatic tissues and activities, resulting in lower reproductive effort. Improved conditions for survivorship, such as good physical conditions or a decrease in predator abundance, have a similar effect by increasing returns expected from investment in soma. Conversely, good conditions for reproduction and/or poor conditions for survivorship result in greater current reproductive effort and decreased future reproductive success.
Expenditure per Progeny
Not all offspring are equivalent. Progeny produced late in a growing season often have lower probabilities of reaching adulthood than those produced earlier — hence, they contribute less to enhancing parental fitness. Likewise, larger offspring may usually cost more to produce, but they are also “worth more.” How much should a parent devote to any single progeny? For a fixed amount of reproductive effort, average fitness of individual progeny varies inversely with total number produced. One extreme would be to invest everything in a single very large but extremely fit progeny. Another extreme would be to maximize total number of offspring produced by devoting a minimal possible amount to each. Parental fitness is often maximized by producing an intermediate number of offspring of intermediate fitness: Hence, the best reproductive tactic is a compromise between conflicting demands for production of the largest possible total number of progeny (r-selection) and production of offspring of the highest possible individual fitness (K-selection).
This trade-off between quantity and quality of offspring can be illustrated with a simple graphical model shown in the following two figures (from Pianka 1976). In the unlikely event that progeny fitness increases linearly with parental expenditure (dashed line A in first figure), fitness of individual progeny decreases with increased clutch or litter size (the lowermost dashed curve A). However, because parental fitness — the total fitnesses of all progeny produced — is flat, no optimal clutch size exists from a parental viewpoint (upper dashed line A in the second figure). If, however, the biologically plausible assumption is made that progeny fitness increases sigmoidally with parental investment, an optimal parental clutch size exists.
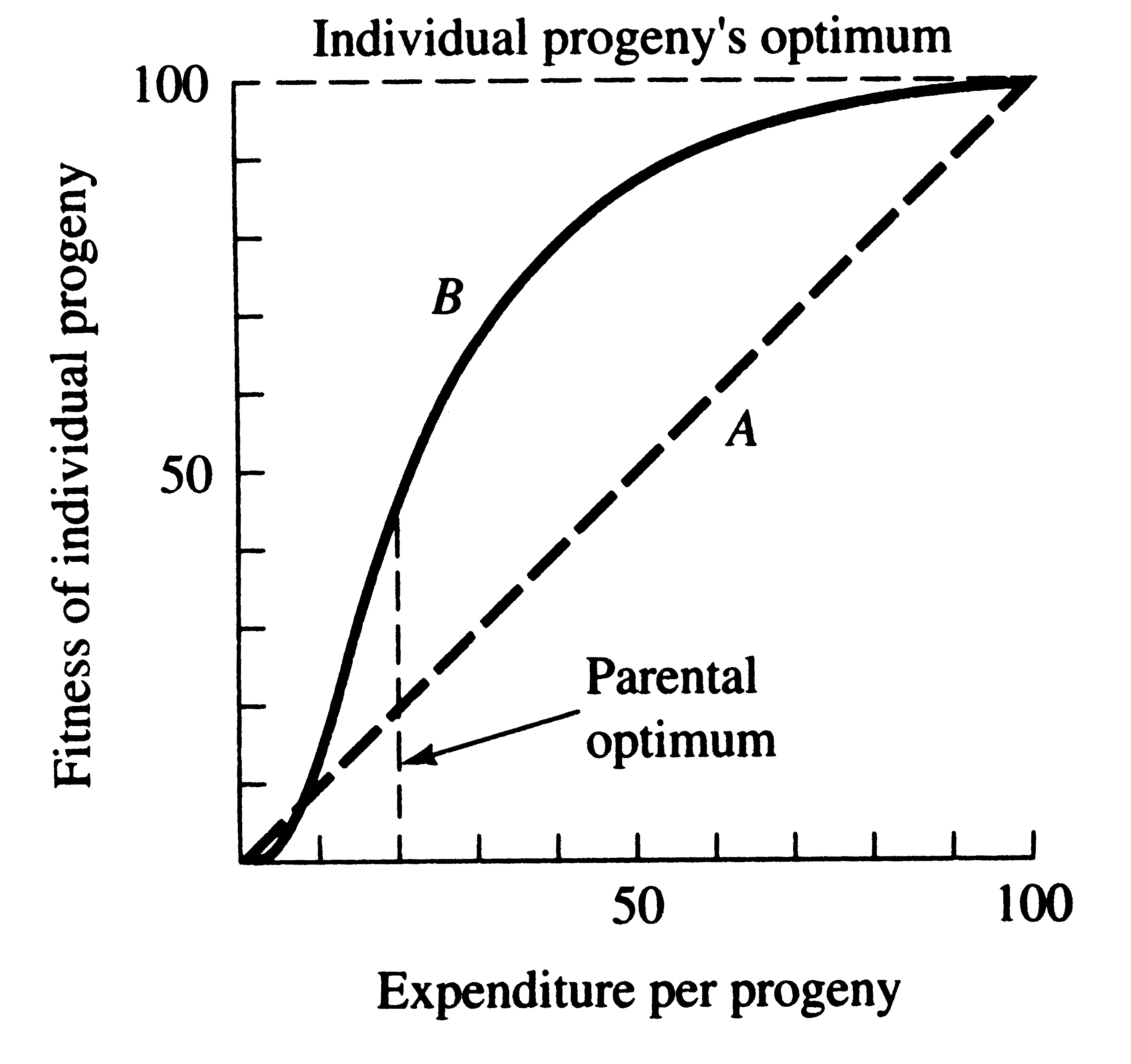
Fitness of an individual progeny generally increases with parental expenditure. Because initial outlays on an offspring usually contribute more to its fitness than subsequent ones, curve B is biologically more realistic than line A. Note that the parental optimum differs from the optimum for individual progeny, setting up a conflict of interests between parents and progeny.
In this hypothetical example, parents that allocate only 20 percent of their reproductive effort to each of five offspring gain a higher return on their investment than parents adopting any other clutch size. Although this tactic is optimal for parents, it is not the optimum for individual offspring, which would achieve maximal fitness when parents invest everything in a single offspring. Hence, a “parent-offspring conflict” exists (Trivers 1974); The exact shape of the curve relating progeny fitness to parental expenditure in a real organism is influenced by a virtual plethora of environmental variables, including length of life, body size, survivorship of adults and juveniles, population density, and spatial and temporal patterns of resource availability. The competitive environment of immatures is likely to be of particular importance because larger, better-endowed, offspring should usually enjoy higher survivorship and generally be better competitors than smaller ones.
Juveniles and adults are often subjected to very different selective pressures. Reproductive effort should reflect environmental factors operating upon adults, whereas expenditure per progeny will be strongly influenced by juvenile environments. Because any two parties of the triumvirate determine the third, an optimal clutch or litter size is a direct consequence of an optimum current reproductive effort coupled with an optimal expenditure per progeny (clutch size equals reproductive effort divided by expenditure per progeny). Of course, clutch size can be directly affected by natural selection as well. Horned lizards are long-lived and relatively K-selected as adults, but their tank-like body form allows them to have a very large reproductive effort -- they produce many tiny offspring which must suffer very high mortality.
Figure. Fitness per progeny (A' and B') and total parental fitness, the sum of the fitnesses of all offspring produced (A and B), plotted against clutch and litter size under the assumptions of the preceding figure. Total investment in reproduction, or reproductive effort, is assumed to be constant. Note that parental fitness peaks at an intermediate clutch size under assumption B; optimal clutch size in this example is five. (From Pianka 1976).
Patterns in Avian Clutch Sizes
Clutch sizes among birds vary from 1 or 2 eggs (albatrosses, penguins, hummingbirds, and doves) to as many as 20 among some non-passerines such as ducks and geese. Among birds, two distinct reproductive tactics are evident: nidicolous and nidifugous. Nidicolous chicks are altricial, hatching out pink and featherless with their eyes closed — such chicks require considerable parental care and stay in the nest for some time before they are fledged. Most passerines are nidicolous (= nest loving). In sharp contrast, chicks of nidifugous (= nest fugitives) non-passerine species are precocial, hatching out with their eyes open and covered with down, fully capable of feeding themselves. In nidifugous species, parental care consists largely of protecting the chicks from predation and teaching them where to feed. A pair of nidicolous blue tits (Parus caeruleus) successfully fledged all the young from a brood of 18 chicks (Lack 1968).
Another dichotomy among birds is determinate versus indeterminate layers. Determinate layers have been genetically programmed to lay a fixed number of eggs and cannot replace lost eggs, whereas indeterminate layers usually can lay almost as many eggs as necessary to fill out their clutch, replacing lost eggs as needed. Chickens are a good example (some white leghorn strains lay more than 200 eggs per year). In a classic experiment with a yellow-shafted flicker woodpecker that normally lays a clutch of 7 or 8 eggs, a researcher removed eggs as fast as a female laid them, leaving one “nest” egg behind so that the female wouldn’t abandon her nest. This female laid 61 eggs over a period of 63 days! Presumably, a female bird feels (“counts”?) the eggs underneath her with her incubating brood patch and some tactile feedback is translated into hormonal changes that terminate egg laying and cause the female bird to become broody and incubate.
Lots of data have now been accumulated demonstrating optimal clutch sizes in birds (for reviews, see Lack 1954 and 1968). These elegant studies show that compared with very small and very large clutches, clutches of intermediate size leave proportionately more offspring that survive to breed in the next generation. This is an excellent example of stabilizing selection. Young birds from large clutches leave the nest at a lighter weight and have a substantially reduced post-fledging survivorship. The optimal clutch apparently represents the number of young for which parents can, on average, provide just enough food. Evidence exists for this in a population of great tits Parus major, which varied their average clutch size from 8 to 12 over a 17-year period, apparently in response to crowding and resulting changes in the density of their major food, caterpillars. Nestling great tits from larger clutches weigh considerably less than those in smaller clutches.
In European starlings, Sturnus vulgaris, clutch size varies from 2 to 8; modal clutch size is four or five eggs, varying seasonally. Although the total number of chicks actually fledged per nest increases monotonically with clutch size, mortality among chicks from large clutches during the first 3 months of life is heavy. As a result, large clutches do not provide a greater return than smaller clutches and a clutch of five eggs constitutes an apparent optimum.
Clutch size in English chimney swifts, Apus apus, varies from 1 to 3 (rarely 4). In these swifts, differential mortality among chicks from clutches of different sizes takes place in the nest before fledging. Swifts capture insect food on the wing and aerial feeding is much better during sunny summers than in cloudy ones. Most chicks from clutches of two leave the nest in sunny years, but only one survives in cloudy years, and the optimal clutch size shifts from 3 to 2. Number of young leaving nest is a function of clutch size in these swifts in England where the success with more than one egg clearly depends on the weather. A polymorphism in clutch size persists with mean fledging success over all years being nearly identical (about 1.7 chicks) for clutches of both 2 and 3.
Seabirds have a long pre-reproductive period (4–5 years) and relatively small clutch sizes (1–3 chicks). Albatrosses do not become sexually mature until they are 5 years old and then they lay only a single egg — Wynne-Edwards (1962) interpreted this as an optimal clutch that produces a net number of young just replacing the parents during their lifetime of reproduction. As such, his “balanced mortality” explanation involves group selection because individual birds do not necessarily raise as many chicks as possible but rather produce only as many as are required to replace themselves. Clearly, a “cheater” that produced more offspring would soon swamp the gene pool. Ashmole (1963) took issue with this interpretation, arguing that albatrosses can successfully raise only one chick. A chick addition experiment performed on the laysan albatross Diomedea immutabilis verified this: an extra chick was added to each of 18 nests a few days after hatching. These 18 nests with 2 chicks were compared with 18 natural “control” nests with just 1 chick. After 3-1/2 months, only 5 chicks survived from the 36 in experimental nests, whereas 12 of the 18 chicks from single-chick nests were still alive. Parents could not find enough food to feed two chicks and most starved to death.
Even within the same widely ranging species, many birds and some mammals produce larger clutches (or litters) at higher latitudes than they do at lower latitudes. Such latitudinal increases in clutch size are widespread and have intrigued many population ecologists because of their general occurrence. The following several hypotheses, which are not mutually exclusive, are among those that have been proposed to explain latitudinal gradients in avian clutch sizes.
Daylength Hypothesis -- During the late spring and summer, days are longer at higher latitudes than at lower latitudes. Diurnal birds therefore have more daylight hours in which to gather food and thus are able to feed larger numbers of young. However, clutch and litter sizes also increase with latitude in nocturnal birds and mammals, which clearly have a shorter period for foraging.
Prey Diversity Hypothesis -- Due to the very high diversity of insects at lower latitudes, tropical foragers are more confused, less able to form search images, and as a result, are less efficient at foraging than their temperate counterparts.
Spring Bloom or Competition Hypothesis -- Many temperate-zone birds are migratory, whereas few tropical birds migrate. During spring months at temperate latitudes, there is a great surge of primary production and insects dependent on these sources of matter and energy rapidly increase in numbers. Winter losses of both resident and migratory birds are often heavy so that spring populations may be relatively small. Hence, returning individuals find themselves in a competitive vacuum with abundant food and relatively little competition for it. In the tropics, wintering migrants ensure that competition is keen all year long, whereas in temperate zones, competition is distinctly reduced during spring months. Thus, because birds at higher latitudes can gather more food per unit time, they can raise larger numbers of offspring to an age at which young can fend for themselves.
Nest Predation Hypothesis -- Tropical habitats house proportionately more predators, both individuals and species, than do temperate ones. Nest failure due to nest predation is extremely frequent in the tropics. Many nest predators locate bird nests by watching and following the parents. Because parents must make more trips to the nest if they have a large clutch, larger clutches should suffer heavier losses than smaller ones. In support of the hypothesis, hole-nesting birds, which are relatively free of nest predators, do not show as great an increase in clutch size with latitude as birds that do not nest in holes. Moreover, on tropical islands known to support fewer predators than adjacent mainland areas, birds tend to have larger clutches than they do in mainland populations.
Predators have been implicated as a factor in the evolution of clutch size even in avian species that do not feed their young (nidifugous birds). In Alaskan semi-palmated sandpipers, Calidris pusilla, ordinary clutches of four eggs fledge an average of 1.74 chicks; but clutches artificially raised to five fledge only one chick, primarily due to heightened predation.
Hazards of Migration -- Latitudinal gradients in clutch or litter size could also be influenced by the trade-off between expectation of future offspring and optimal current investment in reproduction. If hazards of migration or overwintering at high latitudes inevitably result in greater mortality, expectation of life and residual reproductive value will both be reduced at higher latitudes. This in turn would favor an increased effort in current reproduction — and hence larger clutches.
An observation somewhat difficult to reconcile with above hypotheses is that clutch size often increases with altitude. Neither daylength, insect diversity, migratory tendencies, competition, nor predation need necessarily vary altitudinally. Climatic uncertainty, both instability and/or unpredictability, could well result in reduced competition at higher elevations. Optimal clutch size generally involves a compromise between conflicting demands of predator avoidance, competitive ability, and clutch size.
References on Reproductive Tactics
Ashmole, NP (1963) The regulation of numbers of tropical oceanic birds. Ibis 103b: 458-473.
Fisher, RA (1930) The genetical theory of natural selection. Clarendon Press, Oxford.
Harper, JL, PH Lovell, & KG Moore (1970) The shapes and sizes of seeds. Ann. Rev. Ecol. Syst. 1: 327-356.
Lack, D (1954) The natural regulation of animal numbers. Oxford University Press, New York.
Lack, D (1968) Ecological adaptations for breeding in birds. Methuen, London.
Pianka ER (1976) Natural selection of optimal reproductive tactics. Amer. Zool. 16: 775-784.
Pianka ER (2000) Evolutionary Ecology. Sixth Edition. Benjamin-Cummings, Addison-Wesley-Longman. San Francisco.
Trivers, RL (1974) Parent-offspring conflict. Amer. Zool. 14: 249-264.
Wynne-Edwards, VC (1962) Animal dispersion in relation to social behaviour. Oliver and Boyd, Edinburgh.
|