10| Sociality
Use of Space: Home Range and Territoriality
Most habitats consist of a spatial-temporal mosaic of many different, often intergrading, elements, each with its own complement of organisms and other resources. Because of this extensive environmental heterogeneity, an individual's exact location is often a major determinant of its immediate fitness. Members of a prey species that are well protected from their predator(s) in one environmental patch type may be extremely vulnerable to the same predator(s) in another patch. Natural selection, by favoring those individuals that select better microhabitats, should produce a correlation between preference for a given patch type and fitness within it. The density of other individuals in a particular patch, of course, strongly influences the suitability of a given patch. Moreover, because most animals use several to many microhabitats for different purposes and/or at different times of day, the fitness relations of the use of space are usually quite complex.
Organisms can be spaced about the landscape in two extreme ways. They may occur in groups (clumped spatial distributions) or individuals may be evenly spread out (dispersed spatial distributions). Intermediate between these extremes are random spatial distributions, in which organisms are spread randomly over the landscape. Statistical techniques have been developed to quantify the spatial relationships of individuals in a population. One such technique uses the ratio of variance to mean in the numbers of individuals per quadrat. When this ratio is unity, the distribution of organisms in the quadrats fits the Poisson distribution and the organisms are randomly distributed with respect to quadrats. Ratios less than unity indicate dispersion, whereas those greater than unity reflect clumped distributions. Dispersed spatial distributions are generally indicative of competition and K-selection; however, random and clumped distributions, in themselves, indicate little about factors influencing the distribution of the organisms concerned.
Organisms vary widely in their degree of mobility (frequent ecological synonyms are motility and vagility). Some, such as terrestrial plants and sessile marine invertebrates like barnacles, spend their entire adult life at one spot, with their gametes (and/or larvae) being the dispersal stages. Others, like earthworms and snails, although vagile, seldom move very far. Still others, such as the monarch butterfly, some sea turtles and whales, as well as migratory birds, regularly move distances of hundreds to thousands of kilometers during their lifetimes.
Returns on many species of banded birds have shown that even after migrating thousands of kilometers, individuals often return to the same general area where they were raised (this is known as philopatry). Similarly, fruit flies (Drosophila) labeled with radioactive tracers do not usually move very far. Such restricted movements presumably allow individuals to become genetically adapted to local conditions. Two extreme types of populations are distinguished, although once again there are all degrees of intermediates: viscous populations, in which individuals do not usually move very far, and fluid populations, in which individuals cover great distances. In viscous populations there is little gene flow and great genetic variability can occur from place to place, whereas the opposite is true of fluid populations. [Note that kin selection (later in this Chapter) is much more likely to occur in viscous populations than in fluid ones -- colonizing species with high dispersal abilities are likely to have more fluid populations than equilibrium species or climax species.] At a local level, however, inbreeding in a viscous population may lead to reduced genetic variability.
Important differences occur between organisms in the way they use space. The distinction between two-dimensional and three-dimensional patterns of utilization is fundamental (one-dimensional use of space may also be approximated, as along the crest of a sand ridge or the shore of a stream, lake, or ocean). Thus, we find certain ecological similarities between organisms as diverse as plankton, pelagic fish, flying insects, many birds, and bats -- all of which live in a three-dimensional world. The area or volume over which an individual animal roams during the course of its usual daily wanderings and in which it spends most of its time is the animal's home range. Often home ranges of several individuals overlap; home ranges are not defended and are not used to the exclusion of other animals. In contrast, territories are defended and usually are used exclusively by an individual, a pair, a family, or a small inbred group of individuals. Nonoverlapping territories normally give rise to dispersed spacing systems and are invariably indicative of competition for some resource in short supply.
Several different kinds of territories are classified by the functions they serve. Many seabirds, such as gulls, defend only their nest and the area immediately adjacent to it; that is, they have a nesting territory. Some male birds and mammals, such as grouse and sea lions, defend territories used solely for breeding, termed mating territories. By far the most widespread type of territory, however, is the feeding territory, which occurs in a few insects, some fish, numerous lizards, many mammals, and most birds. For territoriality to evolve, some resource must be in short supply and that resource must also be defendable (Figure 10.1).
-
Figure 10.1. Diagrammatic representation of various factors influencing evolution of territoriality.
[From Brown (1964).]
Food items are generally not defendable, since most animals consume their prey as soon as they encounter it. However, the space in which prey occur can often be defended with a reasonable amount of effort. Sometimes even space is not easily defended, especially when food items are very sparse or extremely mobile; under such circumstances, feeding
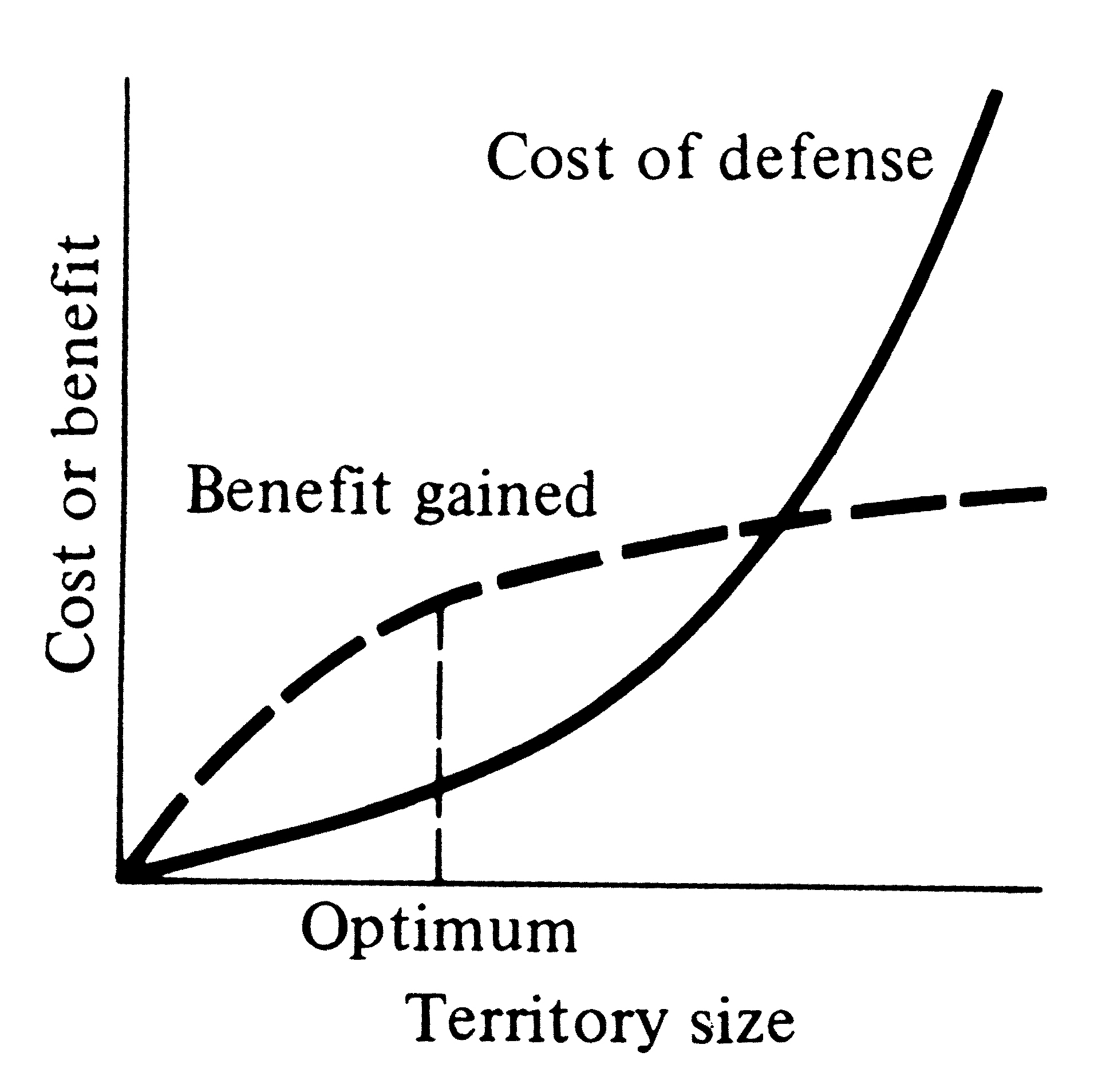 territories cannot be evolved because costs of defense exceed benefits that could be gained (Brown 1964).
territories cannot be evolved because costs of defense exceed benefits that could be gained (Brown 1964).
Figure 10.2. Cost of territorial defense increases monotonically with territory size, but net profits gained decrease above a certain size. At the optimum territory size, the difference between benefit and cost is maximal. An optimal territory size exists where the net benefit, the difference between benefit gained and cost of defense, is greatest.
Often birds that nest in colonies (i.e., seabirds and swallows) defend nesting territories, but because their prey are very mobile and thus indefensible, they have no feeding territories but feed in flocks. Territoriality is particularly prominent in insectivorous and carnivorous birds, probably largely as a result of the energetic economy of flight and their consequent great mobility, which makes territorial defense economically feasible. Typically, males of these birds set up territories during early spring, often even before wintering females return. During this period many disputes over territories occur and there is much fighting among males. Once breeding has begun, however, disputes over boundaries are usually greatly reduced, with males advertising that they are still "on territory" only briefly during the morning and evening hours.
Male ovenbirds recognize individual neighbors by their territorial songs; Weedon and Falls (1959) played tape-recorded songs back in different places and at different rates and times and watched responses of various males to playbacks. When a tape recording of a nonneighbor's call is played from a neighboring territory (after the original neighbor has been removed), a male ovenbird reacts by responding vigorously and singing frequently and loudly. The male thus recognizes the substitution of calls, because the same male does not react strongly to the call of his original neighbor. This technique was used to study the function of the territorial song of white-throated sparrows (Falls 1969); a greater frequency of playback elicits a stronger response, suggesting that highly motivated birds sing more often than birds that are less likely to win a territorial encounter. There are definite advantages to recognizing territorial calls of established neighbors and not reacting strongly to them; it would be a waste of time and energy to respond vigorously to such calls once territorial boundaries have been well established. Both individuals stand only to benefit from a "gentleman's agreement" over where such boundaries lie.
Possession of home ranges and territories also serves other important functions. By becoming familiar with a small local area, an animal can learn (1) when and where food is likely to be found, (2) locations of safe retreats from predators, and (3) in some cases, when and where those predators are likely to be encountered. Thus, resident individuals will normally have distinct advantages over nonresidents (so-called vagrants).
An enormous literature on territoriality exists; the interested reader can find an entry to it through the references at the end of this chapter.
Sex
Most organisms reproduce sexually, although many plants and invertebrates use it only infrequently. The evolutionary origin and selective advantage(s) of sexual reproduction remain major unresolved problems in biology (Williams 1975). Sexual reproduction itself remains an enigma to students of evolution because organisms engaging in sex perpetuate genetic materials of other organisms. In contrast, an organism that reproduces asexually transmits only its own genes to a clone of its offspring genetically identical to itself, thereby leaving twice as many copies of its genes. In effect, reproducing sexually reduces a reproductive organism's contribution to each of her progeny by a full one half, meaning that to perform as well as an asexual, a sexual form must produce twice as many progeny.
Sexual reproduction has arisen more than once. Presumably, it first came into existence in bacteria billions of years ago in primeval seas. In bacteria, sex involves
exchanging genetic material but does not necessarily result in immediate reproduction. A more elaborate form of sexual reproduction arose later in protists that involved evolution of diploidy as well as a complex reduction division (meiosis) and production of haploid gametes with only one set of chromosomes. This form of sexual reproduction has persisted to the present day through the evolution of more complex organisms such as ourselves. Diploidy may have evolved as a sort of "fail-safe" mechanism: when there are two copies of the genetic material, if an error is made, a "good" accurate backup copy still exists.
One plausible idea for the origin of sex is a predation hypothesis. Early organisms that consumed others could have simply adopted some of their prey's genetic material and put it to use to their own ends. According to this view, the predator incorporated some of its prey's loci into its own genome, thereby immediately acquiring the ability to synthesize some useful gene products and hence enhancing its own immediate performance and fitness. As an example, evidence is overwhelming that components of prokaryotes, bacteria and blue-green algae, have been incorporated into eukaryotic higher organisms as cell organelles (chloroplasts and mitochondria).
Once gametes evolved, a distinct advantage to producing two distinct types emerged: one large and nutritious, but sedentary, gamete to support early development (eggs, oocytes, ovules) and another more mobile, but smaller, gamete to carry little more than genetic material (pollen, sperm). Such a specialization of function is superior to the presumed primitive state in which the two gametes are similar in size and function (isogamy). The situation in which gametes adopt different functions is termed anisogamy. Anisogamy gives rise to an asymmetry that results in an interesting fundamental yet inescapable "conflict of interests" between males and females (Trivers 1972), the basis of sexual selection.
Numerous varieties of sexual reproduction exist. Perhaps the finest of all is facultative sexuality, seen in water fleas (Cladocera): these aquatic microcrustaceans abandon sex completely during the relatively constant summer months to form all female clones, with each producing only genetically identical daughters (all females possess two full sets of their mother's chromosomes). With the onset of winter, females produce meiotic eggs that develop into haploid males with only one set of their mother's chromosomes. These males inseminate haploid eggs of females in all clones, which then produce a special, larger, overwintering resting egg via sexual reproduction.
Some organisms are also hermaphroditic, including simultaneous hermaphrodites (in which one individual has both male and female gonads at the same time -- as in many invertebrates such as earthworms and many plants) and sequential hermaphrodites that change sex during their lifetimes. Among certain marine fishes, some species are males when young but then change sex to become females as they grow older and larger, whereas other species of coral reef fish are females when small and become males as they get older and larger. Sex change in such fish is under social control -- if the male is removed, the largest female becomes a male. At least one sequentially hermaphroditic plant species may be able to switch back and forth from being either male or female and vice versa. In the most familiar organisms, most vertebrates and some plants, the sexes are separate.
Sexual processes allow genes in a gene pool to be mixed up each generation and recombined in various new combinations; as such, genetic variability is generated by sexual reproduction. The potential rate of evolution of a sexual population is far greater than that of a group of asexual organisms simply because a variety of beneficial mutations are readily combined into the same individual in a sexual species. But a rapid potential rate of evolution is seldom of as much immediate advantage to an individual organism as is a doubly high rate of reproduction. Sexual reproduction is certainly very basic in diploid organisms and is doubtless an ancient and primitive trait. Considered from an individual's perspective, however, sex is expensive because an individual's genes are thereby mixed with those of another organism and hence each offspring carries only half its genes (i.e., heritability is halved). In contrast, a female reproducing asexually (including parthenogenesis) duplicates only her own genome in each offspring. Even Fisher (1930) suggested that sex could conceivably have evolved for group benefit by way of some non-Darwinian form of group selection. Strangely enough, although many temporary losses of sexuality have been secondarily evolved, relatively few known organisms seem to have completely lost the capacity to exchange their genes with those of other organisms for any geologically long period of time. All female, unisexual species (well known in fish and lizards), are presumably short lived on the geological time scale. (See box "Virgin Birth in Human Females?")
_______________________________________________________________________________________
Virgin Birth in Human Females?
Parthenogenetic reproduction could occur among human females yet remain unnoticed. Indeed, such a woman could have a husband and yet be totally unaware of her own condition. She would have only daughters, each of which would carry only her genes, which would almost certainly increase in the gene pool, at least over the short term. Is there any evidence for this? Claims of reproduction without males are not to be expected from nunneries, but neither have any emanated from prisons where women are kept isolated from men. Parthenogenesis in humans may seem far-fetched, but 50 years ago no one suspected that parthenogenesis could occur in any vertebrate: now all female species have been documented in fish, amphibians, reptiles and birds (all major orders of vertebrates except mammals).
In the mid-1950s, the British medical journal Lancet published an editorial pointing out that it could be difficult to establish suitable criteria for recognition of parthenogenesis in humans. This set into motion a train of events that led to an interesting if too limited scientific examination. The Sunday Pictorial newspaper asked mothers who believed that they had produced a child by virgin birth to come forward. Two different mechanisms exist by which a female could reproduce without contact with a male: (1) budding from somatic cells of the mother or incomplete disjunction during meiosis of gametogenic cells, (2) autofertilization. In the first situation, mother and daughter would be perfect clones, genetically identical (like identical twins). In the second process, the mother would have to produce a sperm that would inseminate her own egg. Mother and daughter would not be genetically identical although the daughter would possess a subset of her mother's genes, possibly being homozygous at some loci where her mother was heterozygous.
The newspaper article unfortunately mentioned that such children would have to be daughters (it would have been interesting to see whether or not any sons were claimed, but, if so, they could not possibly be parthenoforms). Ultimately, 19 women presented themselves along with their daughters as examples of "virgin birth." Eleven of these did not profess that no father existed, but were under the mistaken impression that the search was for a hymen intact after conception (but long since broken in birth).
The remaining eight pairs were examined by Balfour-Lynn (1956), who blood typed mothers and daughters and found antigens present in six daughters that were absent in their mothers, clear evidence of genetic differences. In another pair, the mother had blue eyes and the daughter brown eyes, indicating genetic differences. In the single remaining case, "Mrs. Alpha and daughter," there was apparent genetic identity in blood groups and several other genetically determined traits including electrophoretic analysis of serum. The probability of such a close match between a mother and daughter produced by heterosexual reproduction was less than one chance in a hundred (P < .01).
As a final check, reciprocal skin grafts were carried out. The transplant from daughter to mother was rejected (shed) in about 4 weeks, while the one from mother to daughter remained healthy for 6 weeks before it was removed. Balfour-Lynn (1956) considered these skin graft results obscure, but Beatty (1967) interpreted them to mean that the daughter possessed antigens not present in the mother, and therefore could not be parthenogenetic. Autoimmune responses are known that result in rejection of grafts of one own's skin. Clearly, the jury is still out on this intriguing question: further studies like this one should be undertaken. By now, "Mrs. Alpha's daughter" may well have daughters of her own that could be tested by modern techniques such as DNA fingerprinting.
_______________________________________________________________________________________
One brave evolutionist has concluded that sex is maladaptive in higher vertebrates (Williams 1975, p. 109). Evolutionary benefits of genetic recombination and increased variability must more than offset the disadvantage of one organism perpetuating another's genes. In animals with biparental care, two parents can usually raise twice as many progeny as a single parent, offsetting the cost of sex. One possible advantage to an individual could be that by reproducing sexually, an organism can mix its genes with other desirable genes, thereby enhancing the fitness of its progeny (of course, this can work both ways, for by mating with a less fit partner, an organism would tend to diminish its own fitness). Another idea is that competition between siblings is reduced by the formation of a variety of types under sexual reproduction (in contrast, cloned offspring should interfere strongly with one another because they are genetically identical and hence require similar resources). If heterozygosity in itself confers increased fitness, however, sexual reproduction can clearly be advantageous to individuals. It may be no accident that many parthenoform unisexuals have a biparental origin, arising from the hybridization of two bisexual parental species. Of course, in such a situation, clonal reproduction maintains and perpetuates heterozygosity perfectly, even better than sexual reproduction would.
Sex Ratio
In populations of many dioecious organisms, numbers of males and females are approximately equal. The sex ratio is defined as the proportion of males in the population. To be more precise, we distinguish the sex ratio at conception, or the primary sex ratio, from that at the end of the period of parental care, the secondary sex ratio. The sex ratio of newly independent nonbreeding animals (e.g., as recently fledged birds) is the tertiary sex ratio, whereas that of the older breeding adult population is the quaternary sex ratio (sometimes called the operational sex ratio).
Some have asked "Why have males?" Why are various sex ratios often near equality (i.e., 50:50, or 0.5)? Darwin (1871) speculated that sex ratios of 1:1 might benefit groups by minimizing intrasexual fighting over mates. Other workers have reasoned that since one male can easily serve a number of females, it might "be better for the species" if the population sex ratio were biased in favor of females, because this would increase the total number of offspring produced. Similarly, males are sometimes viewed as supernumerous and therefore "dispensable." Such interpretations invoke naive group selection, and it is preferable to look for an explanation of sex ratio in terms of selection at the level of the individual.
In sexually reproducing diploid species, exactly half the genes
(more precisely, half those on autosomal chromosomes) come from males and half from females each
and every generation no matter what the population sex ratio (Fisher 1930). This statement merely
asserts that every individual organism has a mother and
 a father, but its implications regarding the sex ratio are extensive.
The reproductive success of all females must therefore be equal to
that of all males -- hence, if the numbers of one sex are lower than those of the other sex, then,
on average, an individual of the underrepresented sex leaves more descendants than an individual
of the overrepresented sex. In effect, individuals of the sex in short supply become more valuable. a father, but its implications regarding the sex ratio are extensive.
The reproductive success of all females must therefore be equal to
that of all males -- hence, if the numbers of one sex are lower than those of the other sex, then,
on average, an individual of the underrepresented sex leaves more descendants than an individual
of the overrepresented sex. In effect, individuals of the sex in short supply become more valuable.
![]() Ronald Fisher Ronald Fisher
Fisher concluded that, at equilibrium, an optimal organism should allocate exactly half its reproductive effort to producing progeny of each sex; thus, if each male offspring costs approximately as much to produce as each female offspring, the optimal family sex ratio is near 50:50, provided that the population is in or near equilibrium. Note that Fisher's argument does not depend on competition for mates in any way, as it assumes that each male has the same probability of mating as every other male (likewise all females are assumed to be of equivalent fitness).
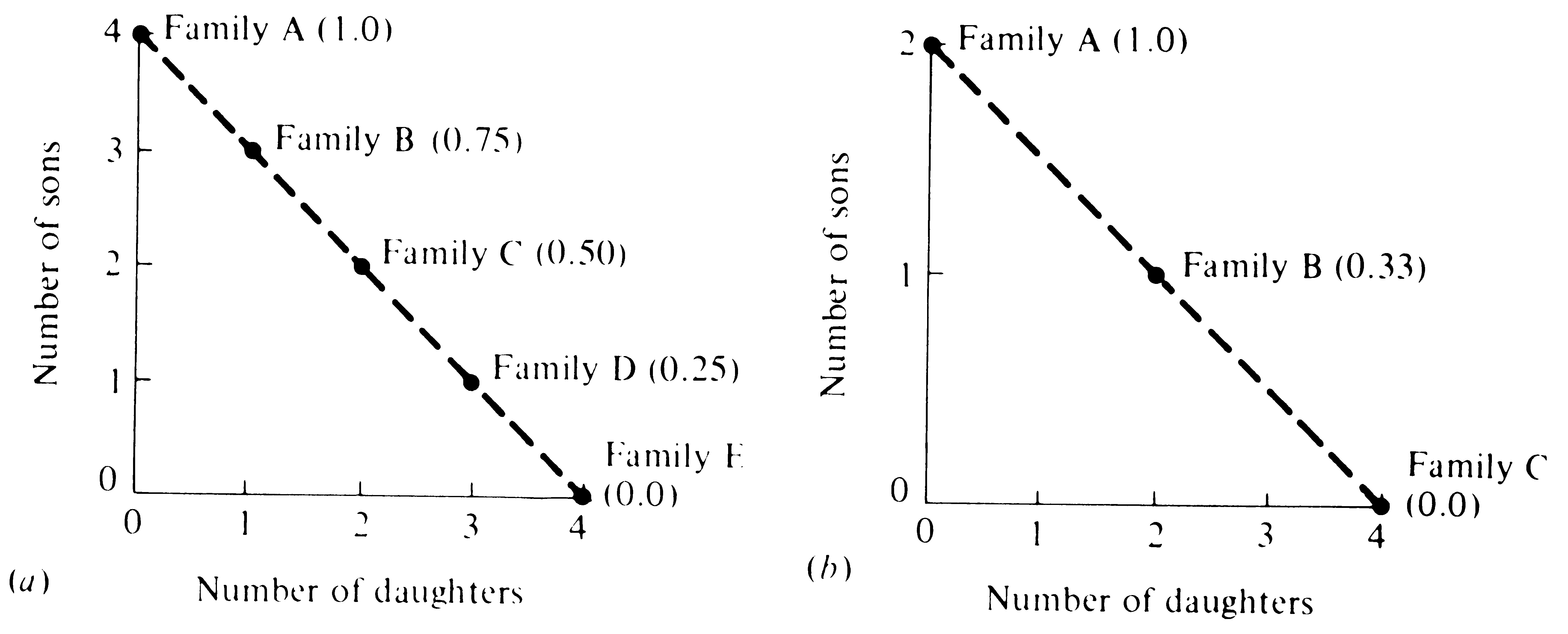
Figure 10.3 and Table 10.1 illustrate Fisher's principle in two hypothetical populations. In the first case, there is no sexual dimorphism in energy demands of progeny and the parental investment argument can be translated directly into numbers; thus, this case leads to an optimal family sex ratio at equilibrium of 0.5. This is not true in the second case, where there is an investment differential such that individual offspring of one sex require twice as much parental expenditure as individuals of the other sex; this case leads to an optimal family sex ratio at equilbrium of either 0.33 or 0.67, depending on which
-
Figure 10.3. Two hypothetical situations illustrating possible family structures without a sexual dimorphism in costs to the parent (a) and with such a sexual differential (b). Parents are assumed to invest a given fixed amount in reproduction. In (a), sons cost parents the same amount as daughters and the optimal family sex ratio (provided that the population is at equilibrium) is 0.50, at which sex ratio, parental investment is equalized on offspring of the two sexes. In (b), male progeny cost their parents twice as much as female progeny, and expenditure on offspring of the two sexes is equalized at a family sex ratio of 0.33 (again, provided that the population at large is near equilibrium). Table 10.1 and the text develop the reasons that parental investment should be divided equally between offspring of each sex when the population is at equilibrium.
sex is more expensive to produce. In both cases, the optimal family sex ratio differs when the population sex ratio deviates from the optimal family sex ratio at equilibrium. In such a circumstance, families producing the sex in deficit (compared to the equilibrium sex ratio) have a selective advantage; this results in excess production of the underrepresented sex, which then forces the population sex ratio to the equilibrium sex ratio.
Table 10.1 Comparison of the Contribution to Future Generations of Various Families in Case a and Case b of Figure 10.3 in Populations with Different Sex Ratios
________________________________________________________________________________
Case a![]() Number of Males Number of Males![]() Number of Females Number of Females
________________________________________________________________________________
Initial population![]() 100 100![]() 100 100
Family A![]() 4 4![]() 0 0
Family C![]() 2 2![]() 2 2
Subsequent population (sum)![]() 106 106![]() 102 102
CA = 4/106 = 0.03773
CC = 2/106 + 2/102 = 0.03846 (family C has a higher reproductive success)
________________________________________________________________________________
Case b![]() Number of Males Number of Males![]() Number of Females Number of Females
________________________________________________________________________________
Initial population![]() 100 100![]() 100 100
Family A![]() 2 2![]() 0 0
Family B![]() 1 1![]() 2 2
Subsequent population (sum)![]() 103 103![]() 102 102
CA = 2/103 = 0.01942
CB = 1/103 + 2/102 = 0.02932 (family B is more successful)
Initial population![]() 100 100![]() 100 100
Family B![]() 1 1![]() 2 2
Family C![]() 0 0![]() 4 4
Subsequent population (sum)![]() 101 101![]() 106 106
CB = 1/101 + 2/106 = 0.02877
CC = 4/106 = 0.03773 (family C is more successful than family B)
Natural selection will favor families with an excess of females until the population reaches its equilibrium sex ratio (below).
Initial population![]() 100 100![]() 200 200
Family B![]() 1 1![]() 2 2
Family C![]() 0 0![]() 4 4
Subsequent population (sum)![]() 101 101![]() 206 206
CB = 1/101 + 2/206 = 0.001971
CC = 4/206 = 0.01942 (family B now has the advantage)
________________________________________________________________________________
Note: The contribution of family x is designated Cx.
Sex ratio is a special case of frequency-dependent selection, which arises whenever the selective value of a trait changes with its frequency of occurrence. Figure 10.4 shows the way in which optimal family sex ratio varies with population sex ratio when the two sexes are equally expensive to produce. This plot is more general in that it shows how optimal investment of a family varies as average expenditure in the population at large changes. Note that when average expenditure on offspring of the two sexes by the entire
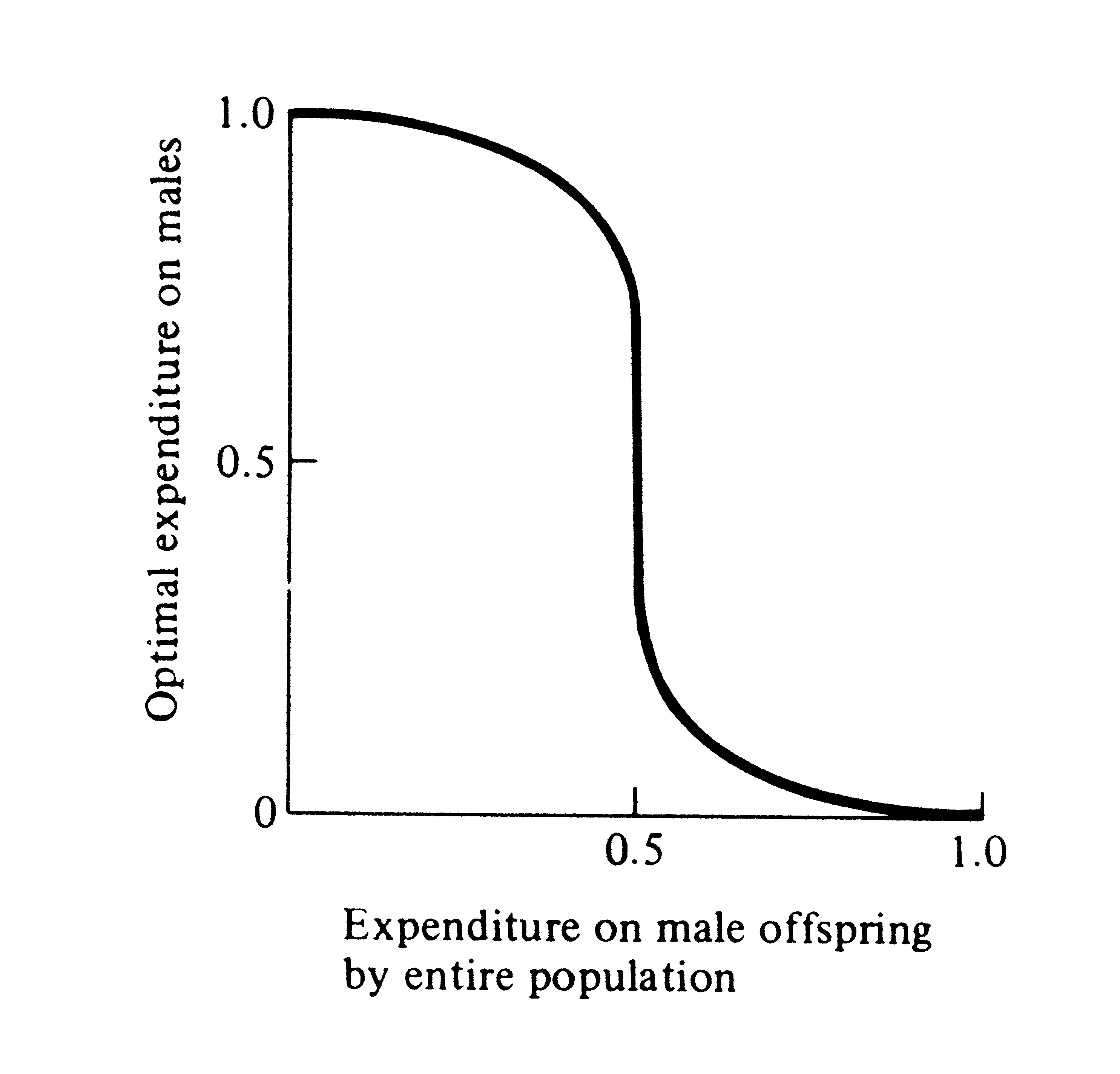 population is near equilibrium (1:1), a relatively broad range of family strategies is near optimal; as overall population expenditure deviates more from this equilibrium, the optimal family tactic rapidly converges on producing families containing only the more valuable underrepresented sex.
population is near equilibrium (1:1), a relatively broad range of family strategies is near optimal; as overall population expenditure deviates more from this equilibrium, the optimal family tactic rapidly converges on producing families containing only the more valuable underrepresented sex.
-
Figure 10.4. Optimal family expenditure on sons
varies
with expenditure on male offspring in the
population at large.
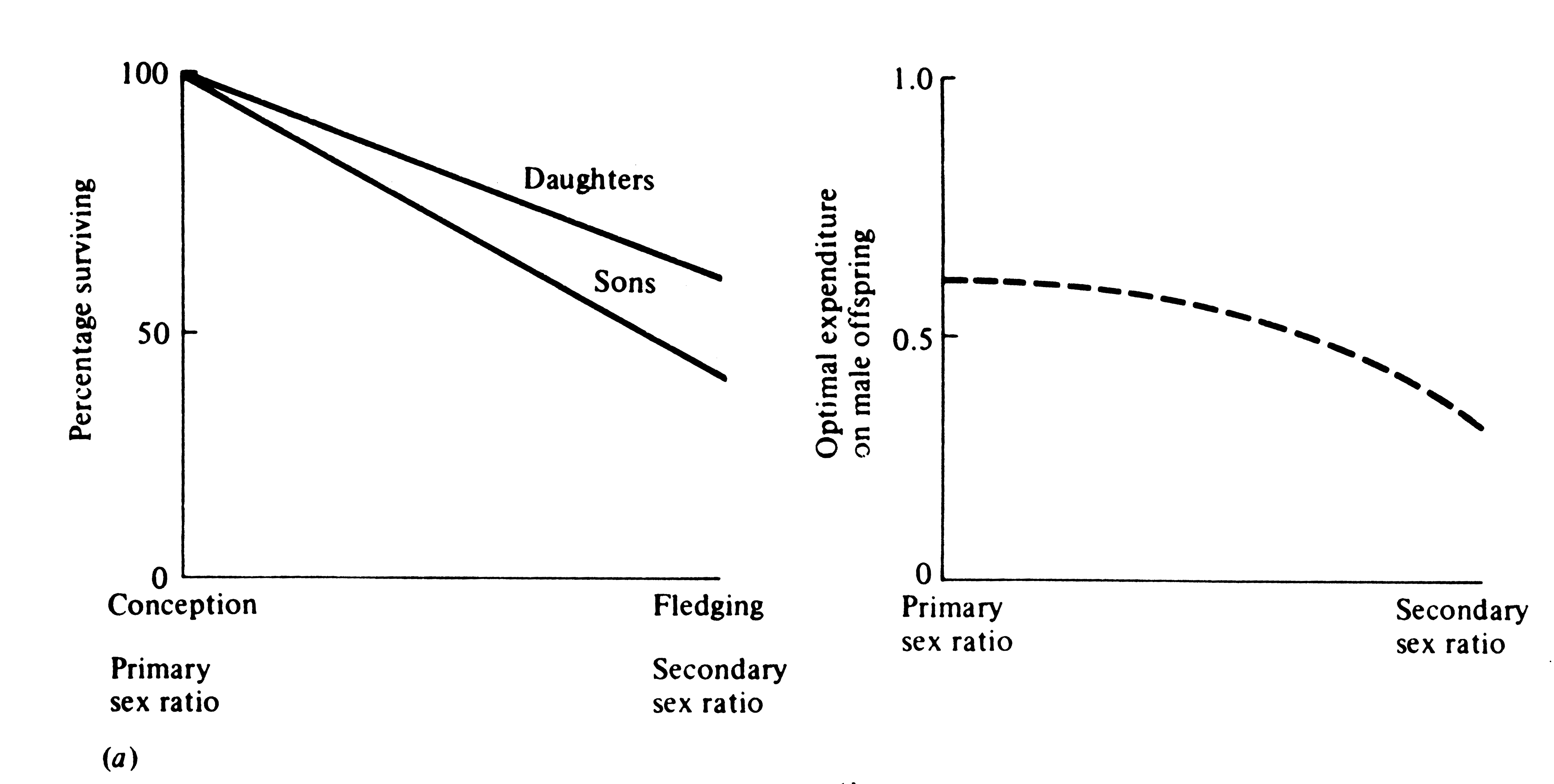
Thus, the only factor that can influence primary and secondary sex ratios is a sexual difference in costs of progeny to the parents. A special case is differential mortality of the sexes during the period of parental care (Figure 10.5). Because differential energetic requirements and differential mortality after this period do not interact with parental expenditure, they cannot directly alter either primary or secondary sex ratios unless their effects are also manifest during the period of parental care. Sexual dimorphisms, or physiological, morphological, and/or behavioral differences between the sexes, are of paramount importance in any discussion of sex ratio. A great variety of ecological factors can influence sexual dimorphism, especially sexual selection and mating systems, which are complexly intertwined (next section).
 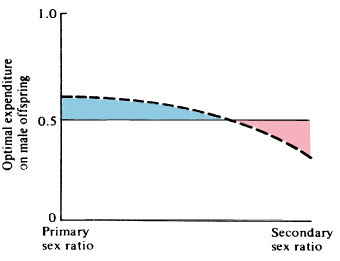
-
Figure 10.5. When there is differential mortality between offspring of the two sexes during the period of parental care, parental expenditure on the two sexes can only be equalized through skewed primary and secondary sex ratios. For example, if sons die off more rapidly than daughters (left panel), the optimal primary sex ratio will be biased toward sons, whereas the optimal secondary sex ratio is biased in favor of daughters (right panel). The line curves because the proportion of males to females decreases more and more rapidly as surviving daughters accumulate.
Parental manipulation of sex ratio has been demonstrated in eusocial insects and in zebra finches (Trivers and Hare 1976; Burley 1981, 1986). In mate choice experiments, zebra finches banded with red or orange bands are preferred over those banded with blue or green bands. In long-term breeding experiments, parental birds with one parent banded with either an attractive or an unattractive leg band fledged more progeny of the same sex or of the opposite sex, respectively (Burley 1986).
Sexual Selection and Mating Systems
Fisher (1958a) quotes an unnamed "modern" biologist as having asked the question, "Of what advantage could it be to any species for the males to struggle for the females and for the females to struggle for the males?" (italics mine). As Fisher points out, this is really a pseudo-question because the fundamental units of natural selection are individuals rather than species. The question thus reflects the attitude of a naive group selectionist. In the next few pages, we see exactly why this is not a biologically meaningful question.
Given that an organism is to mix its genes with those of another individual (i.e., it is to reproduce sexually), just which other individual those genes are mixed with can make a substantial difference. By virtue of associating its genes with "good" genes, an organism mating with a very fit partner passes its own genes on to future generations more effectively than another genetically identical individual (twin) mating with a less fit partner. Thus, those members of any population that make the best matings leave a statistically greater contribution to future generations. As a result, within each sex there is competition for the best mates of the opposite sex; this leads to the intrasexual component of sexual selection. Intrasexual selection usually generates antagonistic and aggressive interactions between members of a sex, with those individuals best able to dominate other individuals of their own sex being at a relative advantage. Often, direct physical battle is unnecessary and mere gestures (and/or various other signals of "strength") are enough to determine which individual "wins" an encounter. This makes some selective sense, for if the outcome of a fight is relatively certain, little if anything can be gained from actually fighting; in fact, there is some disadvantage due to the finite risk of injury to both contestants. Similar considerations apply in the defense of territories.
Maynard Smith (1956) convincingly demonstrated mating preferences in laboratory populations of the fruit fly Drosophila subobscura. Females of these little flies usually mate only once during their lifetime and store sperm in a seminal receptacle. Males breed repeatedly. Genetically similar females were mated to two different strains of males, one inbred (homozygous) and the other outbred (heterozygous), and all eggs laid by these females during their entire lifetimes were collected. A similar total number of eggs were laid by both groups of females, but the percentage of eggs that hatched differed markedly. Females mated to inbred males laid an average of only 264 fertile eggs each, whereas those bred to outbred males produced an average of 1134 fertile eggs per female (and hence produced over four times as many viable offspring). Maynard Smith reasoned that there should therefore be strong selection for females to mate with outbred rather than with inbred males. When virgin females were placed in a bottle with outbred males, mating occurred within an hour in 90 percent of the cases; however, when similar virgins were offered inbred males, only 50 percent mated during the first hour. Both kinds of males courted females vigorously and repeatedly attempted to mount females, but outbred males were much more successful than inbred ones. By carefully watching their elaborate courtship behavior, Maynard Smith discovered that inbred males responded more slowly than outbred males to the rapid side-step dance of females. Presumably as a result of this lagging, females often rejected advances of inbred males and flew away before being inseminated. These observations clearly show that females exert a preference as to which males they will accept. Similar mating preferences almost certainly exist in most natural populations, although they are usually difficult to demonstrate.
In an elegant study of mate choice among captive feral pigeons, Burley and Moran (1979) and Burley (1981b) demonstrated clear mating preferences among these monogamous birds. Pair bonds were first broken and pigeons kept isolated by sex for several months. Then a "chooser" bird was offered a "choice pair" of potential mates of the opposite sex. Pigeons in such a choice pair were tethered to prevent direct physical contact and differed from one another in a given phenotypic aspect such as age, plumage color, or past reproductive experience. Choosers selected mates with reproductive experience over inexperienced birds and tended to reject very old potential mates in favor of younger ones. A clear hierarchy in preference for plumage traits was also evident; "blue" birds were almost invariably preferred over "ash red" birds, and among female choosers, "blue check" males were chosen over "blue bar" males (either blue phenotype was in turn preferred over "ash red").
Over evolutionary time, natural selection operates to produce a correlation between male genetic quality and female preference because those females preferring males of superior genetic quality would associate their own genes with the best males' genes and therefore produce high-quality sons (female quality is correlated with male preference for the same reason). This can be termed the "good genes" or "sexy son" phenomenon. Female preference also enhances male fitness directly, making it difficult to separate female choice from male fitness. Certain female fish appear to copy mating choices of other females (Dugatkin 1996; Schlupp et al. 1994).
As a result of such mating preferences, populations have breeding structures. At one extreme is inbreeding, in which genetically similar organisms mate with one another (homogamy); at the other extreme is outbreeding, in which unlikes mate with each other (heterogamy). Outbreeding leads to association of unlike genes and thus generates genetic variation. Inbreeding produces genetic uniformity at a local level, although variability may persist over a broader geographic region. Both extremes represent nonrandom breeding structures; randomly mating panmictic populations described by the Hardy-Weinberg equation of population genetics lie midway between them. However, probably no natural population is truly panmictic.
Animal populations also have mating systems. Most insectivorous birds and carnivorous birds and mammals are monogamous (although extra-pair copulations do occur), with a pair bond between one male and one female. In such a case, both parents typically care for the young. Polygamy refers to mating systems in which one individual maintains simultaneous or sequential pair bonds with more than one member of the opposite sex. There are two kinds of polygamy, depending on which sex maintains multiple pair bonds. In some birds, such as marsh wrens and yellow-headed blackbirds, one male may have pair bonds with two or more females at the same time (polygyny). Much less common is polyandry, in which one female has simultaneous pair bonds with more than one male; polyandry occurs in a few bird species, such as some jacanas, rails, and tinamous. In some species, a male has several short pair bonds with different females in sequence; typically, each such pair bond lasts only long enough for completion of copulation and insemination. This occurs in a variety of birds (including some grackles, hummingbirds, and grouse) and mammals (many pinnepeds and some ungulates). Finally, an idealized mating system (perhaps, more appropriately, a lack of a mating system) is promiscuity, in which each organism has an equal probability of mating with every other organism. True promiscuity is extremely unlikely and probably nonexistent; it would result in a panmictic population. It may be approached in some invertebrates such as certain polychaete worms and crinoid echinoderms, which shed their gametes into the sea, or in terrestrial plants that release pollen to the wind, where they are mixed by currents of water and air. However, various forms of chemical discrimination of gametes -- and therefore mating preferences -- probably occur even in such sessile organisms.
The intersexual component of sexual selection (that occurring between the sexes) is termed epigamic selection (Darwin 1871). It is often defined as "the reproductive advantage accruing to those genotypes that provide the stronger heterosexual stimuli," but it is also aptly described as "the battle of the sexes." Epigamic selection operates by mating preferences. Of prime importance is the fact that what maximizes an individual male's fitness is not necessarily coincident with what is best for an individual female, and vice versa. As an example, in most vertebrates, individual males can usually leave more genes under a polygynous mating system, whereas an individual female is more likely to maximize her reproductive success under a monogamous or polyandrous system. Sperm are small, energetically inexpensive to produce, and are produced in large numbers. As a result, vertebrate males have relatively little invested in each act of reproduction and can and do mate frequently and rather indiscriminately (i.e., males tend toward promiscuity). Vertebrate females, on the other hand, often or usually have much more invested in each act of reproduction because eggs and/or offspring are usually energetically expensive. Because females have so much more at stake in each act of reproduction, they tend to exert much stronger mating preferences than males and to be more selective as to acceptable mates (the sex that invests most is the choosier, thereby exerting strong pressure on evolution of the opposite sex). Female choice can be an exceedingly powerful force in male evolution, sometimes generating extreme sexual dimorphisms such as that seen in peacocks. By refusing to breed with promiscuous and polygynous males, vertebrate females can sometimes "force" males to become monogamous and to contribute their share toward raising the offspring. In effect, polygyny is the outcome of the battle of the sexes when the males win out (patriarchy), whereas polyandry is the outcome when females win out (matriarchy). Monogamy is a compromise between these two extremes.
Under a monogamous mating system, a male must be certain that the offspring are his own; otherwise, he might expend energy raising offspring of another male (note that females do not have this problem). No wonder monogamous males jealously guard their females against stolen copulations! Nevertheless, cuckoldry is not infrequent (females can sometimes gain a fitness advantage by cheating on their mates -- see discussion of alternative mating tactics). Certainty of paternity is a serious problem for males, but females can be confident that their progeny are indeed their own (female parentage is certain). On the other hand, females mated monogamously are vulnerable to desertion once reproduction is underway.
Let us now examine ecological determinants of mating systems. Some assert that sex ratios "drive" mating systems; under such an interpretation, polygyny arises when males are in short supply and polyandry occurs when there are not enough females to go around. According to this explanation, many species are monogamous simply because sex ratios are often near equality. In fact, quite the reverse is true, with sexual selection and mating systems indirectly and directly determining sexual dimorphisms and hence various sex ratios. In many birds and some mammals, floating populations of nonbreeding males exist. These can be demonstrated by simply removing breeding individuals; typically, they are quickly replaced with younger and less experienced animals (Stewart and Aldrich 1951; Hensley and Cope 1951; Orians 1969b).
Only 14 of the 291 species (5 percent) of North American passerine birds are regularly polygynous (Verner and Willson 1966). Some 11 of these 14 (nearly 80 percent) breed in prairies, marshes, and savanna habitats. Verner and Willson suggested that in these extremely productive habitats, insects are continually emerging and thus food supply is rapidly renewed; as a result, several females can successfully exploit the same feeding territory. However, a similar review of the nesting habitats used by polygynous passerines in Europe (which also constitute about 5 percent of the total number of species) showed no such prevalence toward grassland or marshy habitats (Haartman 1969). Indeed, for elusive reasons, Haartman suggested that closed-in, safe nests were a more important determinant of polygynous mating systems than were breeding habitats. Crook (1962, 1963, 1964, 1965) has suggested that among African weaverbirds, monogamy is evolved when food is scarce and both parents are necessary to raise the young, whereas polygyny evolves in productive habitats with abundant food where male assistance is less essential. This argument, of course, ignores entirely "the battle of the sexes" (epigamic selection).
Polygyny in long-billed marsh wrens was studied in the field in Washington State (Verner 1964). Male wrens build dummy nests (not used by females) scattered around their territories; during courtship, females are escorted around a male's entire territory and shown each dummy nest (this presumably allows females to assess the quality of a male's territory). Some males possessed two females (one male had three), whereas males on adjacent territories had only one female or none at all. Territories of bigamous and trigamous males were not only larger than those of bachelors and monogamous males, but they also contained more emergent vegetation (where female wrens forage). Verner reasoned that a female must be able to raise more young by pairing with a mated male on a superior territory than by pairing with a bachelor on an inferior territory even though she obtains less help from her mate. This has been demonstrated in red-winged blackbirds (Figure 10.6). Verner noted that evolution of polygyny depends on males being able to defend territories containing enough food to support more than one female and her offspring; this condition for evolution of polygyny requires fairly productive habitats.
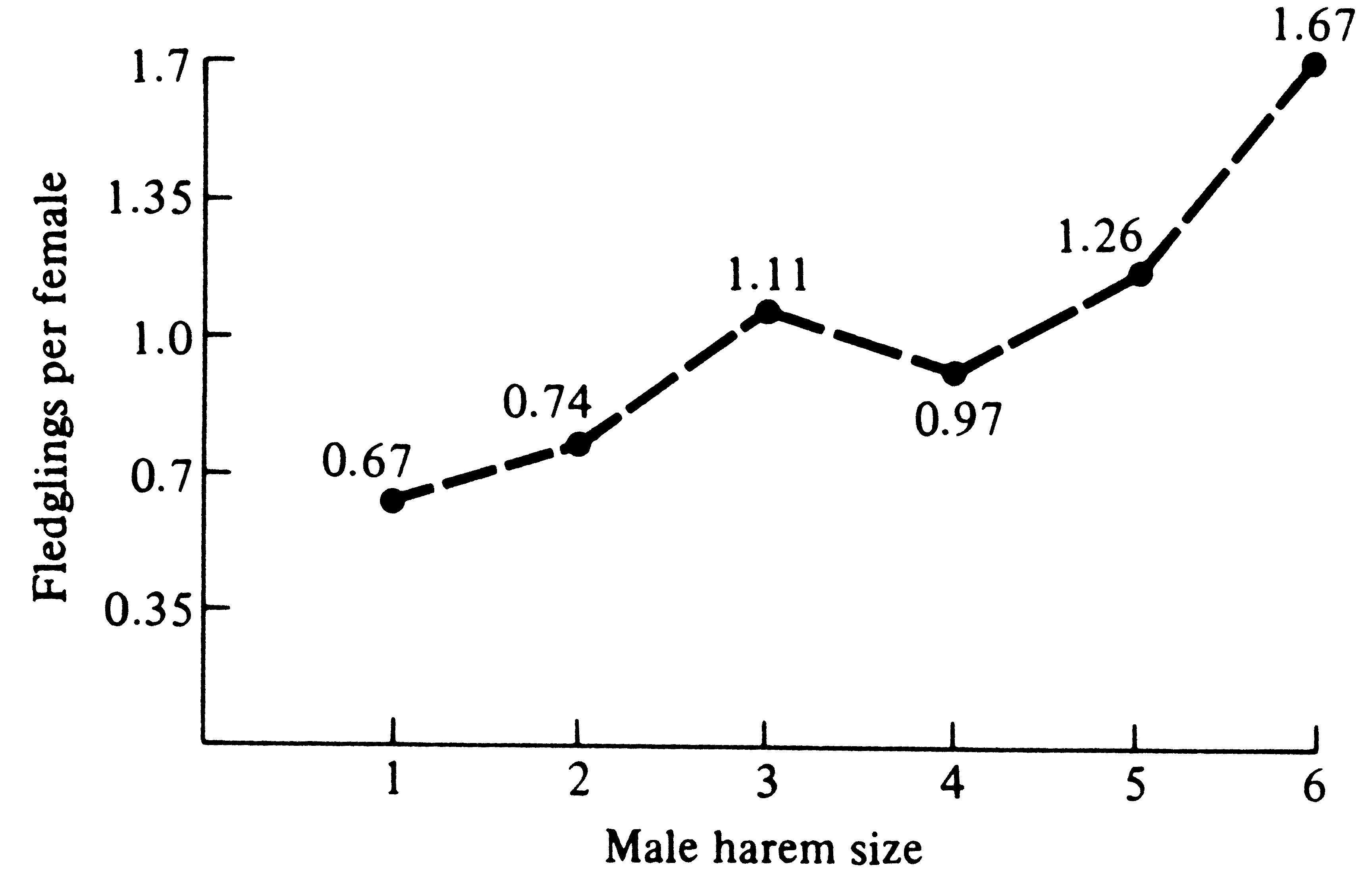
-
Figure 10.6. Reproductive success of female red-winged blackbirds mated with males having different
harem sizes. [After Alcock (1975). Animal Behavior. From data of Holm (1973). Copyright 1973 by the Ecological
Society of America.]
Female wrens are antagonistic toward each other, and as a result, males cannot make a second mating until their first female is incubating; a temporal staggering of females is produced (Verner 1965). Building on Verner's work and studies on blackbirds, Verner and Willson (1966) defined the polygyny threshold as the minimum difference in habitat quality of territories held by males in the same general region that is sufficient to favor bigamous matings by females (Figure 10.7). Polygyny is much more prevalent in mammals than in birds, presumably because in most mammals females nurse their young and, at least among herbivorous species, males can do relatively little to assist females in raising the young (such species typically have a pronounced sexual dimorphism). A notable exception is carnivorous mammals that are often monogamous during the breeding season, with males participating in feeding both female and young (typically sexual dimorphisms are slight in such species). Similarly, most carnivorous and insectivorous birds are monogamous, and males can and do gather food for nestlings. Often, sexual dimorphism in such bird species is slight, and those that are dimorphic are usually migratory (sexual dimorphism may promote rapid pairing as well as species recognition). Birds whose young are well developed at hatching (precocial as opposed to altricial birds) typically have little male parental care and are frequently polygynous, with pronounced sexual dimorphisms.
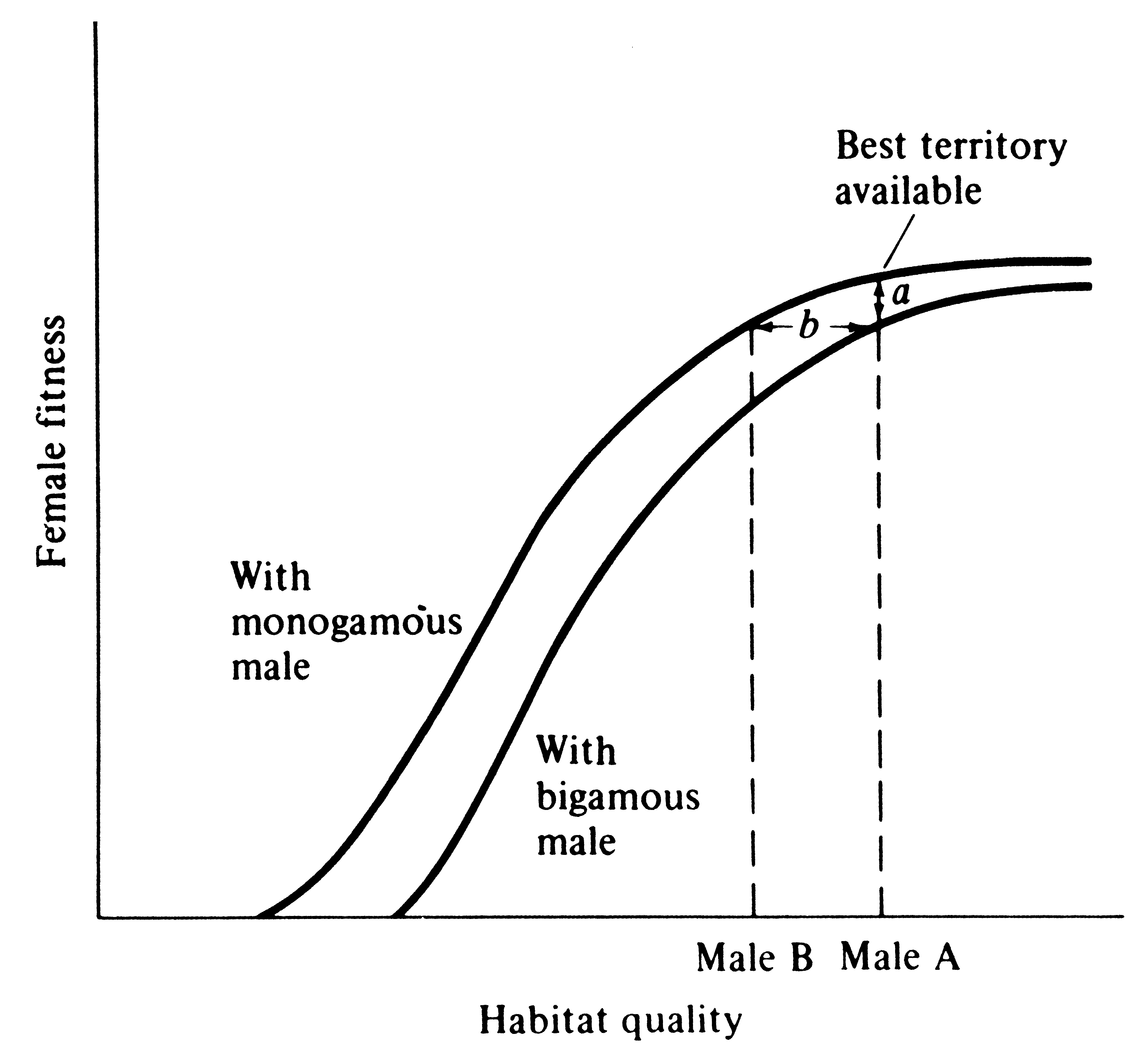
-
Figure 10.7. Graphical model of the conditions necessary for the evolution of polygyny. Reproductive success of females is correlated with the quality of a male's territory: females select mates that give them
the highest individual fitness. Distance a is the difference in fitness between a female mated monogamously and a female mated bigamously in the same environment. Distance b is the polygyny threshold, or the minimum difference in territory quality held by males in the same region that is sufficient to favor bigamous matings by females. [After Orians (1969b).]
Given a population sex ratio near equality and a monogamous mating system, every individual (both male and female) has a substantial opportunity to breed and to pass on its genes; however, with an equal population sex ratio and polygyny, a select group of the fittest males makes a disproportionate number of matings. Dominant battle-scarred males of the northern sea lion, Eumetopias jubata, that have "won" rocky islets where most copulations occur, often have harems of 10 to 20 females.
Under such circumstances, these males sire most progeny and their genes constitute half the gene pool of the subsequent generation. Because those heritable characteristics making them good fighters and dominant animals are passed on to their sons, contests over the breeding grounds may be intensified in the next generation. Only winning males are able to perpetuate their genes. As a result of this intense competition between males for the breeding grounds, intrasexual selection has favored a striking sexual dimorphism in size. Whereas adult females usually weigh less than 500 kilograms, adult males may weigh as much as 1000 kilograms. Sexual dimorphism in size is even more pronounced in the California sea lion, Zalophus californianus, where females attain weights of only about 100 kilograms, whereas males reach nearly 500 kilograms. Among 13 species of these pinniped mammals, sexual size dimorphisms are more pronounced in species that have larger harems (Figure 10.8). Presumably, the upper limit on such a differential size escalation is set by various other ecological determinants of body size, such as predation pressures, foraging efficiency, and food availability. Somewhat analogous situations occur among various polygynous birds. Many species of grouse exhibit multiple short pair bonds, lasting only long enough for copulation, with males displaying their sexual prowess in groups termed "leks."
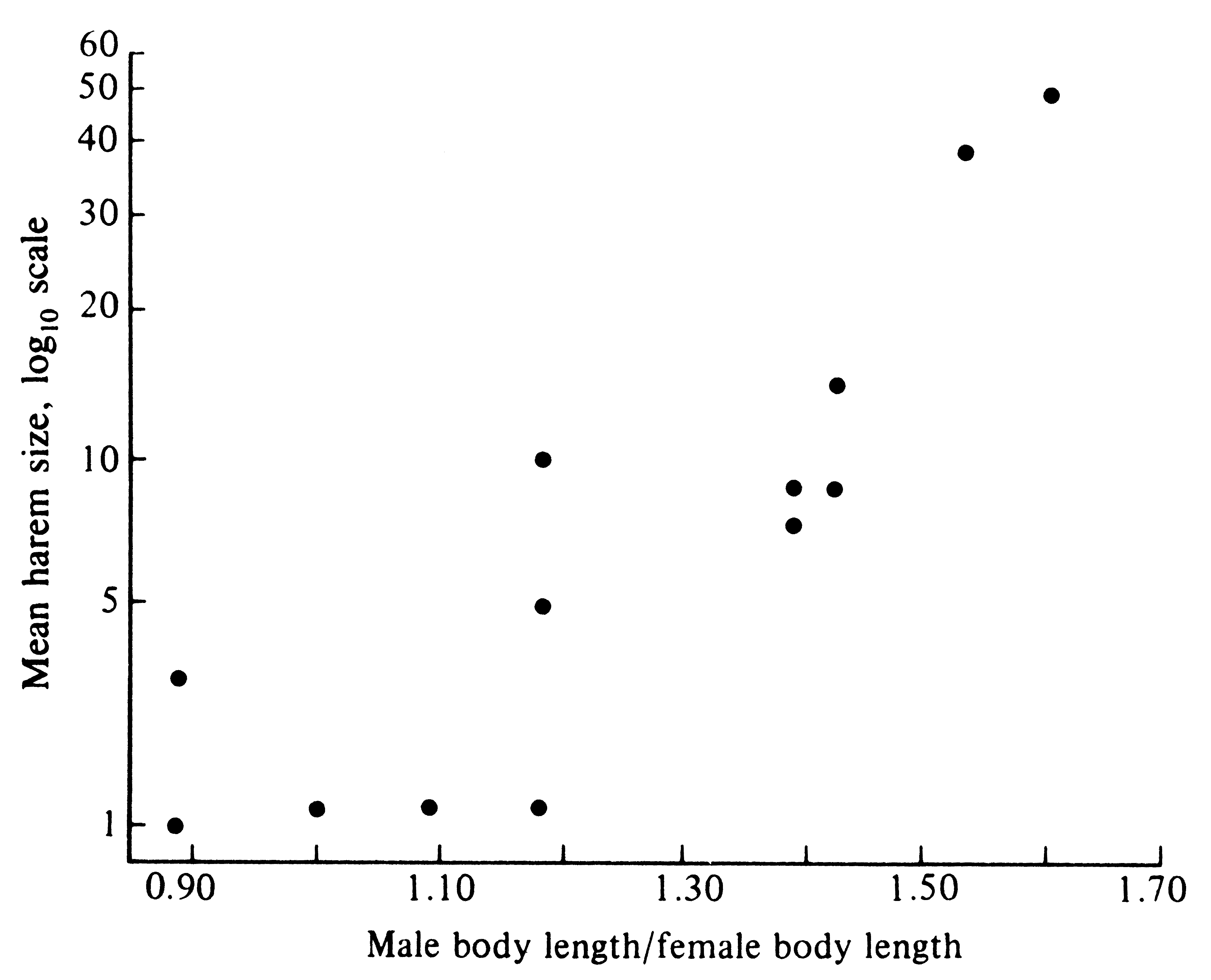
-
![]() Figure 10.8. Average harem size in various species of pinniped sea mammals increases Figure 10.8. Average harem size in various species of pinniped sea mammals increases
![]() with the degree of sexual size dimorphism. [From Trivers (1985) after LeBoufe.] with the degree of sexual size dimorphism. [From Trivers (1985) after LeBoufe.]
Dominant, usually older, males occupy the central portion of the communal breeding grounds and make a disproportionate percentage of matings. Receptive females rush past peripheral males to get to central males for copulation. Strong sexual dimorphisms in size, plumage, color, and behavior exist in many grouse. In addition to intrasexual selection, epigamic selection operating through female choice can also produce and maintain sexual dimorphisms; usually both types of sexual selection occur simultaneously, and it is often difficult to disentangle their effects. Indeed, by choosing to mate with gaudy and conspicuous males, females have presumably forced the evolution of some bizarre male sexual adaptations, such as peacock tails or the long tails of some male birds of paradise (this is known as runaway sexual selection). Certain bower birds have avoided becoming overly gaudy (and hence dangerously conspicuous) by evolving a unique behavioral adaptation; males build highly ornamented bowers that are used to attract and to court females and that signal the male's intersexual attractiveness. Interestingly, male bower birds also demolish and steal from other male's bowers. Frequently, if not usually, the same sexual characteristics (such as size, color, plumage, song, behavior) advertise both intrasexual prowess and intersexual attractiveness. This makes evolutionary sense because an individual's overall fitness is determined by its success at coping with both types of sexual selection, which should usually be positively correlated; moreover, economy of energy expenditure is also obtained by consolidation of sexual signals.
Extravagant male traits such as a peacock's tail could be exploited by females as indicators of the male's ability to survive despite his handicap (Zahavi 1975, 1977; Evans 1991). This "handicap hypothesis" was extended by Hamilton and Zuk (1982), who suggest that females might also assess a male's resistance to parasites by the brightness or elaborateness of his epigamic colors or display.
In some cases, males seem to have evolved to exploit pre-existing sensory biases of females (Andersson 1994). For example, some male insects exploit fragrances of fruit foods as pheromones that attract females. Likewise, calls of male tungara frogs appear to have evolved in response to sensory biases of female frogs (Ryan 1990; Ryan and Keddy-Hector 1992; Ryan and Rand 1990; Ryan et al. 1990). The "sensory exploitation" hypothesis suggests that female mating preferences can evolve without sexual selection and in ways that do not necessarily enhance female fitness. Viewed in this way, males evolutionarily exploit the built-in sensory drive of females, thus increasing their own fitness but not necessarily that of females. Such female "choice" without a fitness advantage for females would seem unlikely to endure if natural selection could operate on variation in female "preference."
Alternative mating tactics exist for both females and males. Males often can increase their reproductive success by extra-pair copulations. A female mated to an average or substandard male can increase the fitness of her progeny by becoming impregnated with the sperm of a superior male (such "stolen copulations" allow the female to produce more attractive sons than she would otherwise, but because her male mate has been cuckolded, his fitness is greatly reduced -- this has led to the evolution of jealousy among males). Still another interesting alternative mating tactic is satellite males. In crickets and frogs, males call to attract mates and females are attracted to superior males by characteristics of their calls. But calling can be hazardous because it also attracts parasites and predators. So-called "satellite" males do not call, but station themselves around calling males and intercept females coming in to mate.
Sexual dimorphisms sometimes serve still another
ecological function, by reducing niche overlap and competition between the sexes (Figure 10.9).
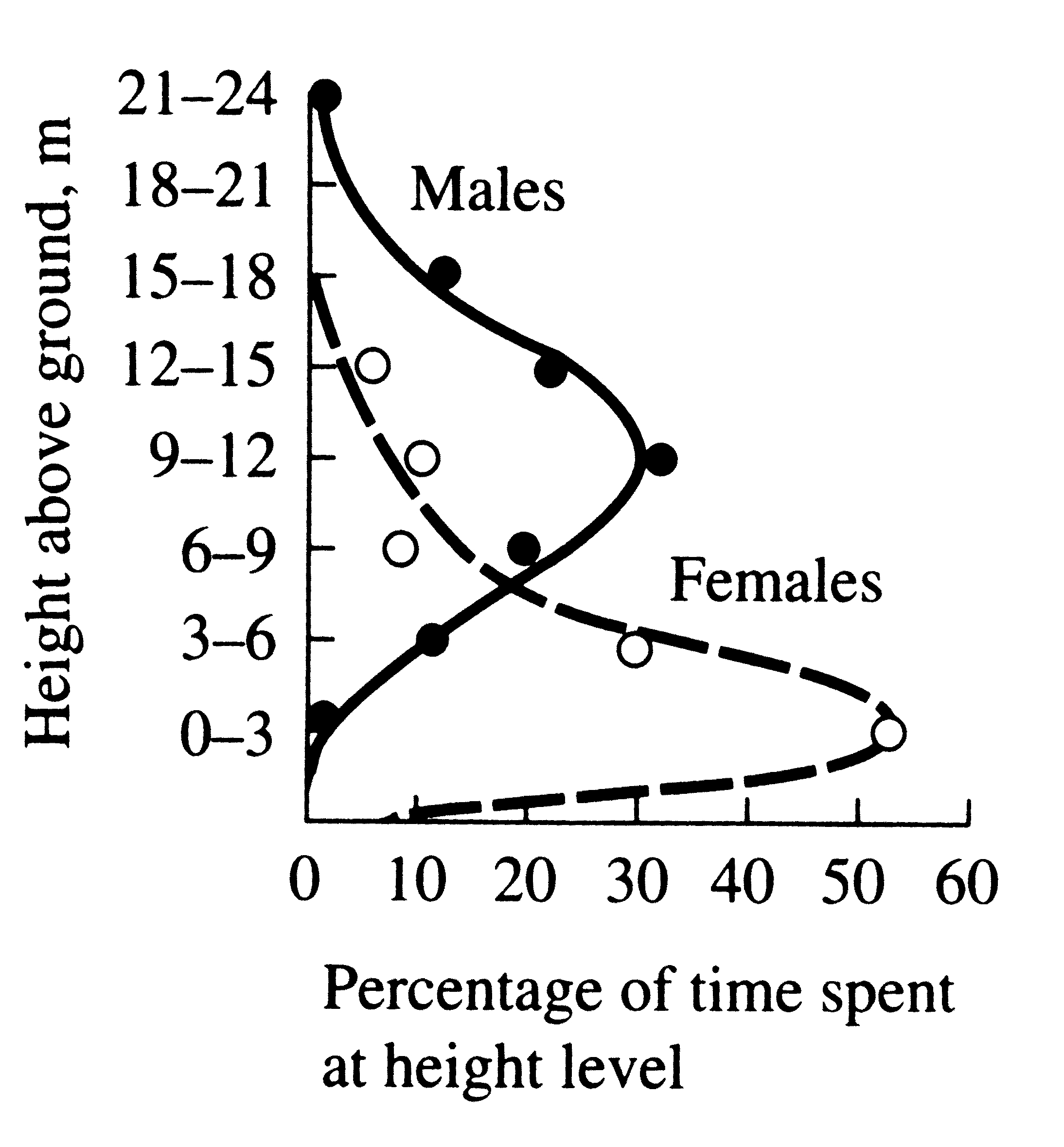 In certain island lizards (Schoener 1967, 1968a) and some birds (Selander 1966), strong sexual dimorphisms in the feeding apparatus (jaws and beaks, respectively) are correlated with differential utilization of food resources which presumably reduces competition between sexes. In certain island lizards (Schoener 1967, 1968a) and some birds (Selander 1966), strong sexual dimorphisms in the feeding apparatus (jaws and beaks, respectively) are correlated with differential utilization of food resources which presumably reduces competition between sexes.
Figure 10.9. Male red-eyed vireos tend to forage higher than females.
[Adapted from P. Williamson (1971). Copyright 1971 by the Ecological
Society of America.]
Fitness and an Individual's Status in Its Population
An organism's fitness is determined by the interaction between its phenotype and the totality of its environment. In K-selected organisms, fitness is determined largely by the biotic environment, especially the individual's status within its own population; however, fitness of r-selected organisms may often depend less on the biotic environment and be more strongly influenced by the physical environment.
Compared with breeding individuals, members of a nonbreeding floating population have very low immediate fitnesses (although their fitness rises if they are able to breed later). Even within a breeding population, various individuals may often differ substantially in fitness; for instance, among long-billed marsh wrens, males vary greatly in their individual fitness, with bigamous and trigamous males being more fit than monogamous ones. Variance in the reproductive success of males is typically much greater than that of females, providing the context for intense sexual selection. Predators tend to crop mainly the "excess" members of bobwhite quail and muskrat populations (Errington 1956, 1963); presumably, resident prey individuals know their own territories and home ranges -- making them more difficult to capture than vagrant individuals with a less stable status in the population.
Social Behavior and Kin Selection
A wide variety of ecological phenomena have been interpreted as having been evolved for the benefit of the population rather than for the benefit of individuals. Clutch size, sex ratio, and sexual selection have already been discussed; predator alarm calls and so-called "prudent predation" are discussed in Chapter 12, and "selection at the level of the ecosystem" is briefly considered in Chapter 14. Another broad area latent with opportunities for interpretation of events and phenomena as having arisen for the advantage of a group is social behavior. Why, for example, does a worker honey bee sacrifice her own reproduction for the good of the colony? As is well known, a worker bee will even give up her own life in defense of the hive. To find the probable answer to this question, we must first develop some basic considerations and definitions.
True altruism occurs only when an individual behaves so that it suffers a net loss while a neighbor (or neighbors) somehow gains from its loss (Table 10.2). Kin selection (see below) occurs when an individual actually gains more than it loses (due to the advantage the individual obtains in perpetuating its genes among its neighbors who are relatives). Thus it is pseudoaltruistic behavior. True altruistic behavior, in which an individual actually loses while its neighbor gains, is virtually unknown (except, perhaps, in humans); moreover, group selection is necessary for its evolution. Obviously selfish behavior will always be of selective advantage; the problem is to explain the occurrence of apparently altruistic behavior such as that of the worker honey bee.
Table 10.2. Four Possible Situations Involving an Individual's Behavior and Its Influence on a Neighbor
__________________________________________________________________
![]() Neighbor(s) Gain Neighbor(s) Gain ![]() Neighbor(s) Lose Neighbor(s) Lose
__________________________________________________________________
Individual Gains ![]() Pseudo-altruistic behavior Pseudo-altruistic behavior![]() Selfish behavior Selfish behavior
![]() (kin selection) (kin selection) ![]() (selected for) (selected for)
__________________________________________________________________
Individual Loses ![]() True altruistic behavior True altruistic behavior ![]() Mutually disadvanta- Mutually disadvanta-
![]() (counterselected) (counterselected) ![]() geous behavior geous behavior
![]() (counterselected) (counterselected)
_________________________________________________________________
The very existence of social behavior implies that individuals living in cooperating groups are in fact leaving more genes in the population gene pool than hermits (Hamilton 1964). Thus, social behavior may be expected to evolve when distinct advantages are inherent in group participation. To develop his thesis, Hamilton defines kin selection as selection operating between closely related individuals to produce cooperation. Selection will favor genes that promote pseudoaltruistic behavior between relatives when rnb - c > 0 (Hamilton's "rule"), where r is a coefficient of relatedness, n is the number of relatives benefited, b is benefit received by each recipient, and c is the cost suffered by the donor. Such behaviors are most likely to be advantageous when multiple relatives benefit but only one donor suffers a cost. As an extreme example, an individual should theoretically sacrifice its own life if it can thereby save the lives of more than two siblings, each of which shares half its genes. Such behavior furthers its own genotype even more effectively than living to reproduce itself. This is not true altruistic behavior because the individual making the "sacrifice" actually gains more than it loses. Kin selection operates at a much more subtle level. Closely related relatives are much more likely to benefit from such pseudoaltruistic behavior than distantly related ones; for the latter to occur, loss to the benefactor should be very small and/or number of distant relatives benefited must be large. Thus, the condition necessary for evolution of such pseudoaltruistic behavior is that total gain(s) to relative(s) must exceed the loss to the pseudoaltruist. Parental care is, of course, a special case of kin selection.
Here again, ideas were foreshadowed by Fisher (1930) in his consideration of the evolution of distastefulness and warning coloration in insects. Many distasteful or even poisonous insects, especially the larvae of some moths and butterflies, are brightly colored; vertebrate predators, especially birds, quickly learn not to eat such warningly colored insects. Fisher noted the difficulty of explaining the origin of this gaudy coloration since new ultraconspicuous mutants would be expected to suffer the first attacks of inexperienced predators and therefore be at a selective disadvantage to less gaudily colored genotypes. He suggested that benefits to siblings on the same branch accruing under a gregarious family system could favor evolution of warning coloration; hence, such ultraconspicuous mutants would be "pseudoaltruists." Fisher even made his argument quantitative by noting that although each sibling shares only half the individual's genes, their numbers ensure that the total number of shared genes benefited greatly exceeds the number destroyed in the individual's own genome. Such a gene for gaudiness raises inclusive fitness, but it cannot spread unless it is already present among relatives: hence the mutant has to have already spread before it can confer a selective advantage.
Two orders of insects are of particular interest because they have evolved eusociality, namely the hymenoptera and isoptera. Members of the insect order Hymenoptera, which includes ants, bees, wasps, and hornets, often form colonies and exhibit apparent altruistic social behavior. Social behavior is thought to have arisen independently many times among hymenoptera, which have a peculiar haplodiploid genetic system; males are produced asexually and are haploid. Thus, all sperm produced by any given male are genetically identical (barring somatic mutation) and carry that male's entire genome. (Males have no father but do have a grandfather.) Females are normal diploids, with each ovum carrying only one-half their genomes. In some hymenopteran species, a queen is thought to mate just once during her entire lifetime and store one male's sperm in a spermatotheca; thus, all her progeny have the same father. A result of this strange genetic system is that sisters are more closely related to one another than mothers are to their own daughters; the former share three-fourths of their genes, the latter only one-half. Hence, one would predict that worker bees should help raise their sisters to reproductive maturity in preference to mating themselves, which in fact they often do. Interestingly, workers can and sometimes do lay haploid male-producing eggs. Males share fewer genes with both siblings and progeny and never work for the benefit of the colony.
Honey bees mate during nuptial flights in the air. Multiple matings by females have been shown to occur by releasing drones with marked sperm. A phenomenon known as sperm precedence occurs in many insects, where the first sperm in is the last sperm out (the last male to copulate gets the first progeny). The implications of multiple mating on the relatedness among colony members merit consideration. Relatedness will remain high as long as the queen is using a given male's sperm, but relatedness will diminish when she switches between the sperm of two males. Bees have long been known to have periods of unrest during which queens are killed and replaced by one of their own royal brood. Such "mutinies" would be expected to occur precisely when queens are switching between the sperm of two different drones (this prediction has not yet been demonstrated conclusively).
Termites (order Isoptera) also form highly organized colonies, complete with a queen and "king," various secondary reproductives, and both male and female workers with distinct castes. But because both sexes of termites have a normal diploid genetic system, the kin selection argument appears to be inadequate to explain the evolution of sociality in termites. Several hypotheses have been offered for this apparent evolutionary enigma. One involves cyclic inbreeding (Bartz 1979, 1980): According to this argument, the primary king and queen in a termite colony are unrelated to one another, but each is itself a highly homozygous product of intense inbreeding. As a result, both copies of alleles at both the maternal and paternal genomes are virtually identical. Such a situation would result in a very high degree of relatedness among their progeny since each offspring receives almost exactly the same set of genes. Termites are highly dependent on one another for continual replenishment of their intestinal protozoa. (These endosymbionts, which produce the cellulases that enable termites to digest wood, are lost with each molt and must be replenished continually during the lifetime of an individual. Socialty assures that a recently molted termite has ready access to being reinoculated from its many nest mates.) Presumably, a pair of termites (the king and queen) maximize their own reproductive success by producing many nonreproductive progeny (workers), which in turn allow production of many successful reproductive offspring (new kings and queens). However, natural selection among workers should operate to release them from such parental "control." Doubtlessly, termites also enjoy other advantages from group cooperation, such as protection from predators and the elements (note that this is also an advantage for hymenopterans).
A detailed long-term study of helpers at the nest in colonially nesting white-fronted bee-eaters in Kenya (Emlen and Wrege 1988, 1989) demonstrated that helpers tended to assist close relatives much more often than distant or nonrelatives (Table 10.3), providing strong evidence for kin selection.
Table 10.3 Helpers at the Nest in White-Fronted Bee Eaters in Kenya
_____________________________________________________________________
Breeders ![]() r* r*![]() Number of Cases Number of Cases![]() % Cases % Cases
_____________________________________________________________________
Father x Mother![]() 0.5 0.5![]() 78 78![]() 44.8 44.8
Father x Stepmother![]() 0.25 0.25![]() 17 17![]() 9.8 9.8
Mother x Stepfather![]() 0.25 0.25![]() 16 16![]() 9.2 9.2
Son x Nonrelative![]() 0.25 0.25![]() 18 18![]() 10.3 10.3
Brother x Nonrelative![]() 0.25 0.25![]() 12 12![]() 6.9 6.9
Grandfather x Grandmother![]() 0.25 0.25![]() 5 5![]() 2.9 2.9
Half brother x Nonrelative![]() 0.13 0.13![]() 3 3![]() 1.7 1.7
Uncle x Nonrelative![]() 0.13 0.13![]() 2 2![]() 1. 1 1. 1
Grandmother x Nonrelative![]() 0.13 0.13![]() 1 1![]() 0.6 0.6
Grandson x Nonrelative![]() 0.13 0.13![]() 1 1![]() 0.6 0.6
Great grandfather x Nonrelative![]() 0.13 0.13![]() 1 1![]() 0.6 0.6
Nonrelative x Nonrelative![]() 0.0 0.0![]() 20 20 ![]() 11.5 11.5
Total![]() 174 174![]() 100.0 100.0
_____________________________________________________________________
* r = coefficient of relatedness.
Source: Adapted from Emlen and Wrege (1988).
Another form of pseudoaltruism, termed "reciprocal altruism" (Trivers 1971), does not require genetic affinity or kin selection to operate. In reciprocal altruism, some behavioral act incurs a relatively minor loss to a donor but provides a recipient with a large gain; thus, two entirely unrelated animals can both benefit from mutual assistance. An example that could perhaps be explained by reciprocal altruism is the posting of sentinels. A sentinel crow spends a brief time period sitting in a tree watching for predators while the rest of the flock forages; in turn it receives continual sentinel protection from the remainder of the flock during the much longer period of time during which it forages. (Such crows may not be sentinels at all but may merely have had their fill of food to eat; their squawk could be a signal intended to inform the predator that it has been detected -- see also Chapter 15.) Reciprocity is, of course, absolutely essential for evolution and maintenance of this sort of altruistic behavior unless kin selection is also operating to promote it. Trivers (1971) suggested that our altruistic and cheating tendencies are in conflict and that reciprocal altruism could well constitute the basis for such things as friendship and play, gratitude and sympathy, loyalty and betrayal, guilt, dislike, revenge, trust and suspicion, dishonesty and hypocrisy. Indeed, there could actually be an evolutionary basis for our sense of justice! Certainly a clan member who didn't pay his dues would have been more likely to have been expelled from the clan than one who did his share.
Cooperation could evolve because the "future can cast a long shadow back on the present" (Axelrod 1984) -- the "tit for tat" strategy is to cooperate on the first interaction and after that to copy what the other player did on its previous move. Note that this can result in cooperation if one's neighbor is cooperative, but can also result in noncooperation if neighbors fail to cooperate. Tit for tat with forgiveness is a stable evolutionary state.
Children are 100 times more likely to be abused or killed by a step-parent than by their genetic parents (Daly and Wilson 1999), strongly suggesting that kin selection operates in humans. Cruel step-parents are not a myth, but are very real.
Kin selection and reciprocal altruism are appealing concepts that facilitate explanation of the evolution of social behavior by natural selection at the level of individuals. However, neither mechanism is readily verified by observation. Future empirical work in these areas, although difficult, will be of great interest.
The Evolution of Self-Deceit
Recent studies with young children have shown that the leaders among children are those who can lie more convincingly. Interestingly, the correlation between leadership and ability to be dishonest did not hold among adult women, although it does among adult males. These startling new discoveries have obvious implications in politics!
Deceit of others is fairly straightforward but self-deceit is an extremely interesting phenomenon worth closer scrutiny. We like to think that we perceive the world around us accurately. In a series of experiments, voices of human subjects were taped and played back to subjects who were wired to a polygraph "lie detector." The polygraph measures electrical conductance across the skin's surface based on perspiration. Our skins, and presumably our subconscious minds, virtually always recognize tape recordings of one's own voice accurately. However, these experiments demonstrated two different forms of self-deception. Sometimes, the conscious response of a subject to hearing his/her own voice is "No, that's not me," but electrical conductance in their own skin shows otherwise (the subconscious recognizes one's own voice). Other times, a subject asserts "Yes, that's me!" to the voice of another person, but skin conductance indicates that the subconscious knows otherwise (i. e., the truth). People who fail to recognize their own voices and who project themselves into another person's voice typically have poor self-images. Presumably, these people were being quite "honest" and genuinely felt that they were giving correct answers. In both situations, the person's conscious mind was deceiving itself while the subconscious mind retained accurate information.
What possible adaptive value could such complex self-deception have? The rather startling possibility is that self-deceit makes one a more effective "liar," enabling one to persuade other humans of some misinformation (imagine the benefits in politics and in litigation!).
Indeed, the evolution of the subconscious mind itself would seem to be a necessary precursor for self-deceit to even become possible. What the subconscious mind actually does with its veritable treasure trove of accurate perceptions remains an open question. My guess is that such information is exploited to maximize the reproductive success of individuals (without their own "knowledge")! The profound implications of self-deceit for politics are disturbing.
Selected References
Use of Space: Home Range and Territoriality
Brown (1964, 1969); Brown and Orians (1970); C. R. Carpenter (1958); F. L. Carpenter (1987); Falls (1969); Howard (1920); Hutchinson (1953); Kohn (1968); McNab (1963); Menge (1972a); Morse (1971); Orians and Horn (1969); Orians and Willson (1964); Pielou (1969); C. C. Smith (1968); Tinbergen (1957); Weedon and Falls (1959).
Sex
Bull (1983); Bull and Harvey (1989); Charnov (1982); Darwin (1871); Fisher (1930); Fricke and Fricke (1977); Shapiro (1980); Stearns (1986); Williams (1971, 1975).
Sex Ratio
Burley (1981, 1986); Charnov (1982); Darwin (1871); Fisher (1930, 1958a); Hamilton (1967); Kolman (1960); Maynard Smith (1976); Verner (1965); Werren and Godfray (1995).
Sexual Selection and Mating Systems
Andersson (1994); Bayliss (1978, 1981); Blumer (1979); Burley (1977, 1981a, b); Burley and Moran (1979); Crook (1962, 1963, 1964, 1965, 1972); Cronin (1991); Daly (1979); Darwin (1871); Davies (1992); Dawkins and Carlisle (1976); Diamond (1988); Downhower and Armitage (1971); Dugatkin (1996); Emlen (1968b); Emlen and Oring (1977); Erckmann (1983); Evans (1991); Evans and Hatchwell (1992); Fisher (1930, l958a); Fricke and Fricke (1977); Grammar and Thornhill (1994); Gross (1994, 1996); Gross and Shine (1981); Haartman (1969); Hamilton (1961); Hamilton and Zuk (1982); Handford and Mares (1985); Hensley and Cope (1951); Houde and Endler (1990); Holm (1973); Howard (1974); Hrdy (1981); Kirkpatrick (1982); Kirkpatrick and Ryan (1991); Kolman (1960); Lack (1968); Lightbody and Weatherhead (1987, 1988); Loehle (1997); Margulis and Sagan (1986); Maynard Smith (1956, 1958, 1971); Orians (1969b, 1972, 1980b); Payne (1979); Perrone and Zaret (1979); Pleszczynska (1978); Ralls and Harvey (1985); Rice and Holland (1997); Ridley (1978); Ryan (1985, 1990, 1997); Ryan and Keddy-Hector (1992); Ryan et al. (1998); Schoener (1967, 1968a); Schlupp et al. (1994); Selander (1965, 1966, 1972); Shapiro (1980); Smouse (1971); Stewart and Aldrich (1951); Thornhill (1976, 1986); Thornhill and Alcock (1983); Trivers (1972, 1985); Trivers and Willard (1973); Verner (1964, 1965); Verner and Engelsen (1970); Verner and Willson (1966); Werren et al. (1980); Whittingham et al (1992); Wiley (1974); Williams (1966a, 1971, 1975); Willson and Burley (1983); Willson and Pianka (1963); Wittenberger (1976); Xia (1992); Zahavi (1975, 1977); Zahavi and Zahavi (1997).
Fitness and an Individual's Status in Its Population
Errington (1946, 1956, 1963); Fretwell (1972); C. C. Smith (1968); Verner (1964, 1965); Verner and Engelsen (1970); Wellington (1957, 1960).
Social Behavior and Kin Selection
Alexander (1974); Axelrod (1984); Axelrod and Hamilton (1981); Bartz (1979, 1980); Brown (1966, 1975); Cole (1983); Crook (1965); Daly and Wilson (1999); Dawkins (1976); Eberhard (1975); Emlen and Wrege (1988, 1989); Evans (1977); Fisher (1930, 1958a); Greenberg (1979); Hamilton (1964, 1967, 1970, 1971, 1972); Hardin (1982); Hoogland (1980); Horn (1968b);Hughes (1988); Maynard Smith (1964); Michod (1982); Myles and Nutting (1988); Oster and Wilson (1979); Price and Maynard Smith (1973); Sherman (1977); Sober and Wilson (1998); Thorne (1997); Trivers (1971, 1974, 1976, 1985); Trivers and Hare (1976); Wallace (1973); Wiens (1966); E. O. Wilson (1971, 1975); Wynne-Edwards (1962).
The Evolution of Self-Deceit
Gur and Sackeim (1979); Trivers (1985).
|

