15| |Predation and Parasitism
Predation
Predation is often readily observed and easily studied, and neither its existence nor its importance in nature is doubted. It is directional in the sense that one member of the pair (the predator) benefits from the association while the other (the prey) is affected adversely. In contrast, competition is a symmetric process in that both species are affected adversely and, where possible, each tends to evolve mechanisms whereby the relationship with the other is avoided.
Individual predators that are better able to capture prey should have more resources at their disposal and should therefore normally be more fit than those that are less proficient at capturing prey. Hence, natural selection acting on the predator population tends to increase the predator's efficiency at finding, capturing, and eating its prey. However, members of the prey population that are better able to escape predators should normally be at a selective advantage within the prey population. Thus, selection on the prey population favors new adaptations that allow prey individuals to avoid being found, caught, and eaten. Obviously, these two selective forces oppose one another; as the prey become more adept at escaping from their predators, the predators in turn evolve more efficient mechanisms for capturing them.
Hence, in the evolution of a prey-predator relationship, the prey evolves so as to dissociate itself from the interaction while the predator continually maintains the relationship. Long-term evolutionary escalations of this sort have resulted in some rather intricate and often exceedingly complex adaptations. Consider, for instance, the complex social hunting behavior of lions and wolves; the long sticky tongues and accurate aim of some fish, toads, and certain lizards; the folding fangs and venom-injection apparatus of viperine snakes; spiders and their webs; the deep-sea angler fish; and snakes such as boas that suffocate their prey by constriction. Other examples include the rapid and very accurate strikes of predators as diverse as praying mantids, dragonflies, fish, lizards, snakes, mammals, and birds. Prey have equally elaborate predator escape mechanisms, such as the posting of sentinels, predator alarm calls, background color matching, and thorns. Many prey organisms recognize their predators at some distance and employ appropriate avoidance tactics well before the predator gets close enough to make a kill; this behavior in turn has forced many predators to hunt by ambush.
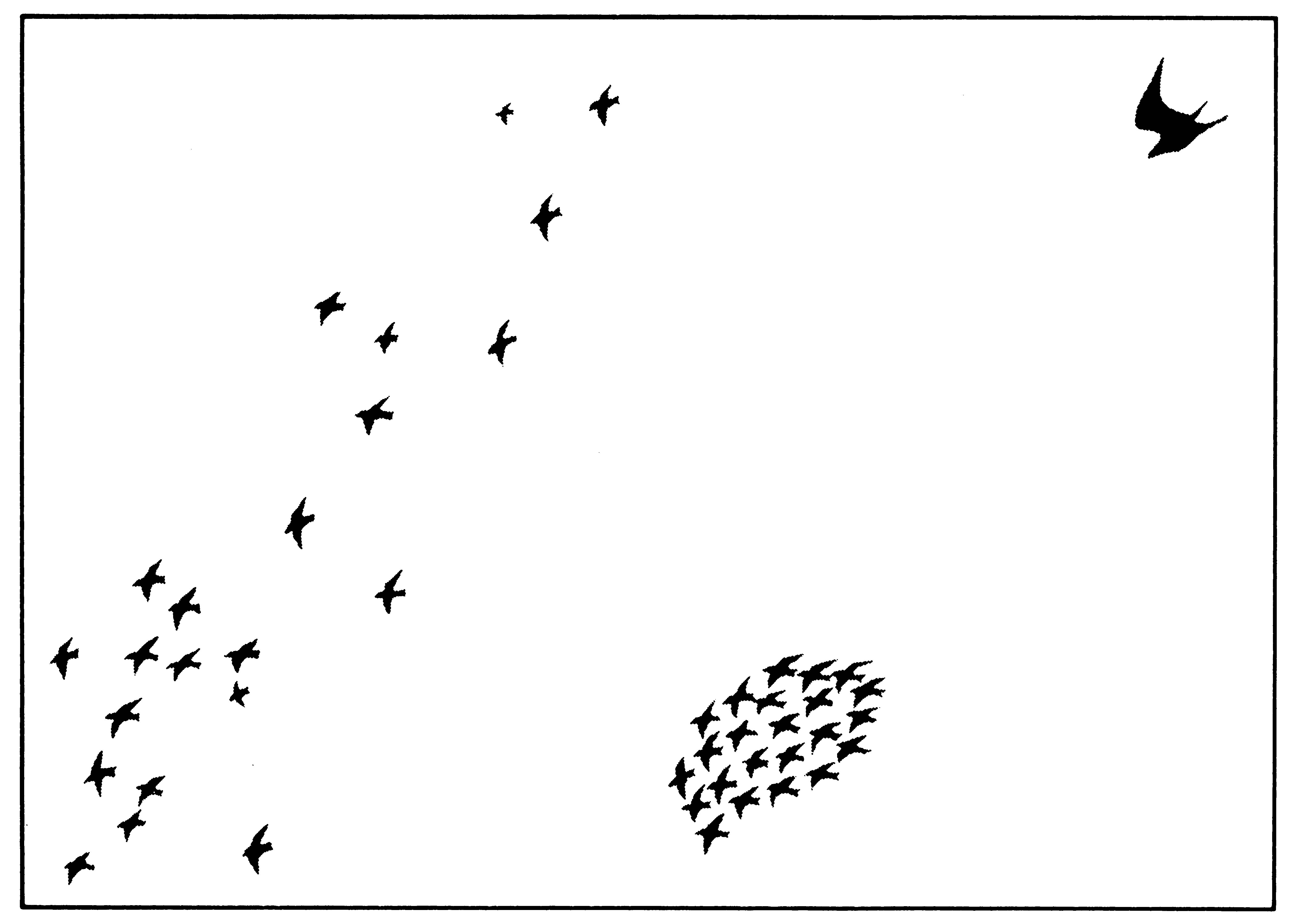
One of my favorite examples of the joint adaptations of a predator and its prey is provided by the starling and the peregrine falcon. The peregrine is a magnificent bird-eating hawk whose hunting behavior must be seen to be fully appreciated. These falcons take other birds as large as themselves; nearly all prey is captured on the wing and in the air. Peregrines have exceedingly keen vision; foraging individuals climb high up into the sky and move across country. When a potential prey is sighted flying along below, the peregrine closes its wings and dives or "stoops." To make its ambush most effective, the falcon often "comes out of the sun" at its prey. Diving peregrines have been estimated to reach speeds of over 300 kilometers/hour (nearly 100 meters/second!). Most prey are killed instantly by the sudden jolt of the peregrine's talons. Large prey are allowed to fall to the ground and eaten there, but smaller items may be carried away in the air. (Little wonder that falconers and their dogs find it extremely difficult to get small birds to fly when a peregrine is "waiting on" overhead! On occasion, game birds allow themselves to be overtaken on the ground by dogs in preference to taking to the air and risking the falcon's deadly stoop.) Starlings normally fly in loose flocks, but when a peregrine is sighted, often at a considerable distance, they quickly assume a very tight formation (Figure 15.1). Tight flocking is a response specific to the peregrine and is not employed
-
Figure 15.1. Starlings normally fly in loose flocks (left), but when under attack by a peregrine falcon, they fly as close together as possible (right).
with other hawks. Falcons are much less likely to attack a tight flock than a single bird; indeed, "stragglers" slightly out of the starling flock formation are often taken by peregrines. Presumably, the falcon itself could be injured if it were to stoop into a tight flock. Thus, even a predator as effective as the peregrine falcon has had its hunting efficiency impaired by appropriate behavioral responses of its prey.
There is an important difference between predation on animals and predation on plants. In most animals, predation is an all-or-none proposition in that the predator kills the prey outright and consumes most or all of it; however, when plants are eaten, usually only a portion of the plant is consumed by its predator. Hence, predation on plants (herbivory) is more like parasitism among animals. But even partial predation must often reduce a prey individual's ability to survive and/or to reproduce. Because of this fundamental difference, though, selective pressures on animals to avoid being eaten may be stronger than they are on plants. Nevertheless, plants have evolved elaborate antipredator devices.
Predator-Prey Oscillations
In terms of their population dynamics, plus-minus interactions are perhaps most interesting because they display a natural tendency to oscillate (May 1973, 1981; Toft 1986). However, these interactions can be stabilized by allowing certain sorts of realistic self-damping to occur. The theory of predation has lagged behind that for competition; perhaps its asymmetry makes it more difficult to model. Lotka (1925) and Volterra (1926a, b, 1931) wrote a simple pair of predation equations:
![]() dN1/dt = r1N1 - p1N1N2 dN1/dt = r1N1 - p1N1N2 ![]() (1) (1)
![]() dN2/dt = p2N1N2 - d2N2 dN2/dt = p2N1N2 - d2N2 ![]() (2) (2)
where N1 is prey population density, N2 is population density of the predator, r1 is the instantaneous rate of increase of the prey population (per head), d2 is the death rate of the predator population (per head), and p1 and p2 are predation constants. The term p1N1 represents the functional response, describing the consumption response of individual predators to changes in prey density, a constant in these simple equations. The p2N1 term in equation (2) represents the numerical response, describing the way in which prey are converted into new predator individuals. Each population is limited by the other and there are no self-limiting density effects (that is, no second order N1 or N2 terms). Thus, in the absence of the predator, the prey population expands exponentially and the rate of increase of the prey population is potentially unlimited. The product of the densities of the two species, N1N2, reflects the number of contacts between them; after multiplication by the constant, p2, this term becomes the maximal rate of increase of the predator population (p2N1N2 ). The same term multiplied by the constant, p1, appears with a negative sign in the prey equation and acts to decrease the rate of growth of the prey population.
-
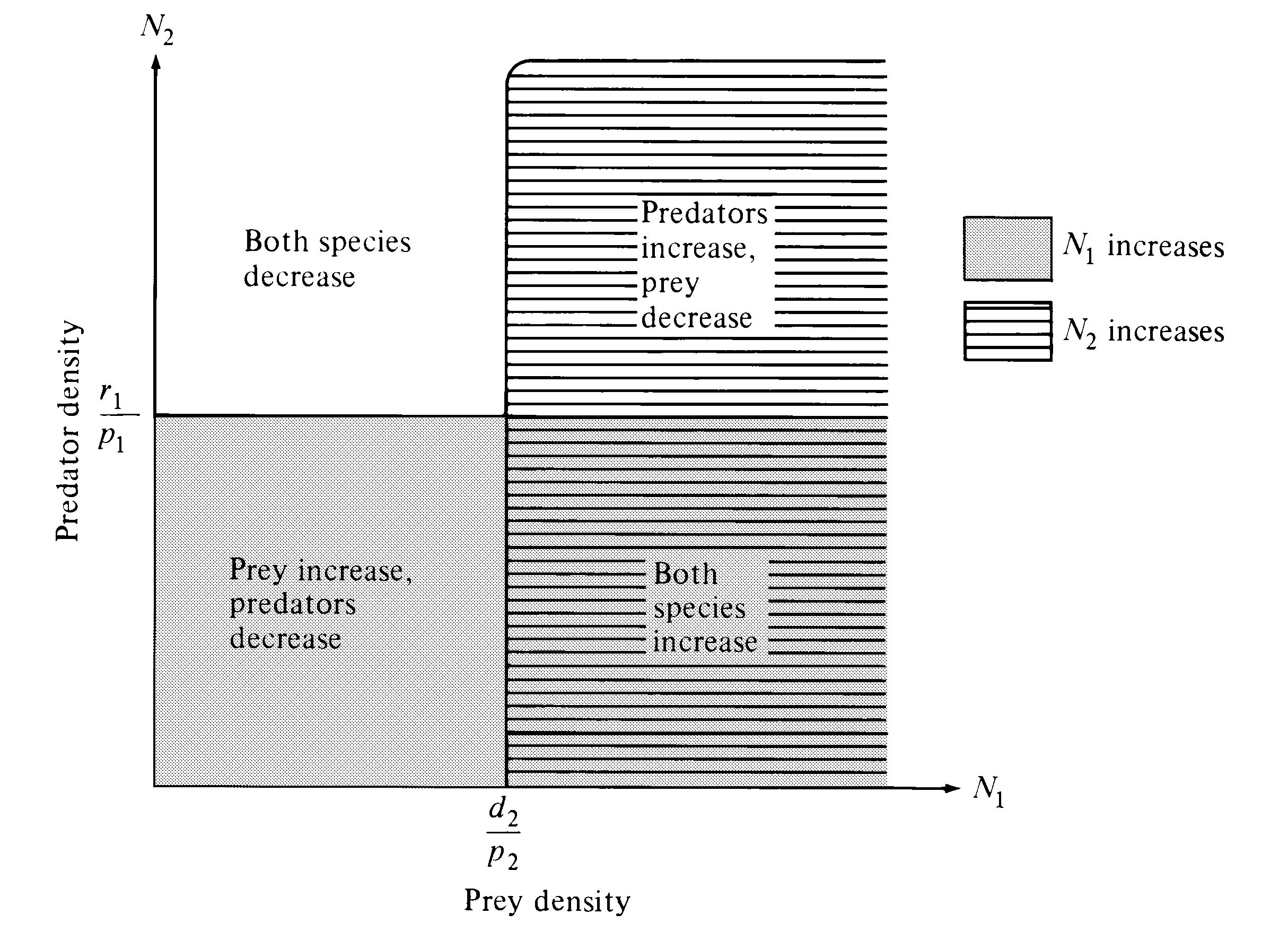
Figure 15.2. Prey and predator isoclines for the Lotka-Volterra prey-predator equations (see text).
The equations are solved by setting dN/dt equal to zero, factoring out the appropriate N to get the actual rate of increase, and setting this ra equal to zero. These algebraic manipulations show that the prey reaches an equilibrium population density when the predator's density is r1/p1; similarly, the predator is at equilibrium when the prey's density is d2/p2 (Figure 15.2). Thus, each species' isocline corresponds to a particular (constant) density of the other species, and again there is no self-damping term such as the -zN2 term in the competition equations. Below some threshold prey density, predators always decrease, whereas above that threshold they increase; similarly, prey increase below a particular predator density but decrease above it (Figure 15.2). A joint equilibrium exists where the two isoclines cross, but prey and predator densities do not converge on this point. Rather, any given initial pair of densities results in oscillations of a certain magnitude. Initial densities near the joint equilibrium point result in repeating oscillations of low amplitude; initial densities farther from the joint equilibrium point generate oscillations of greater amplitude. Thus, this pair of differential equations has a periodic solution, with the population densities of both prey and predator changing cyclically and out of phase over time. The amplitude of the fluctuations depends on initial conditions. Mathematically, a system of such repeating and undamped oscillations is termed neutrally stable. Neutral stability probably does not exist in the biological world since most individuals and populations encounter either self-regulation or density-dependent feedback.
-
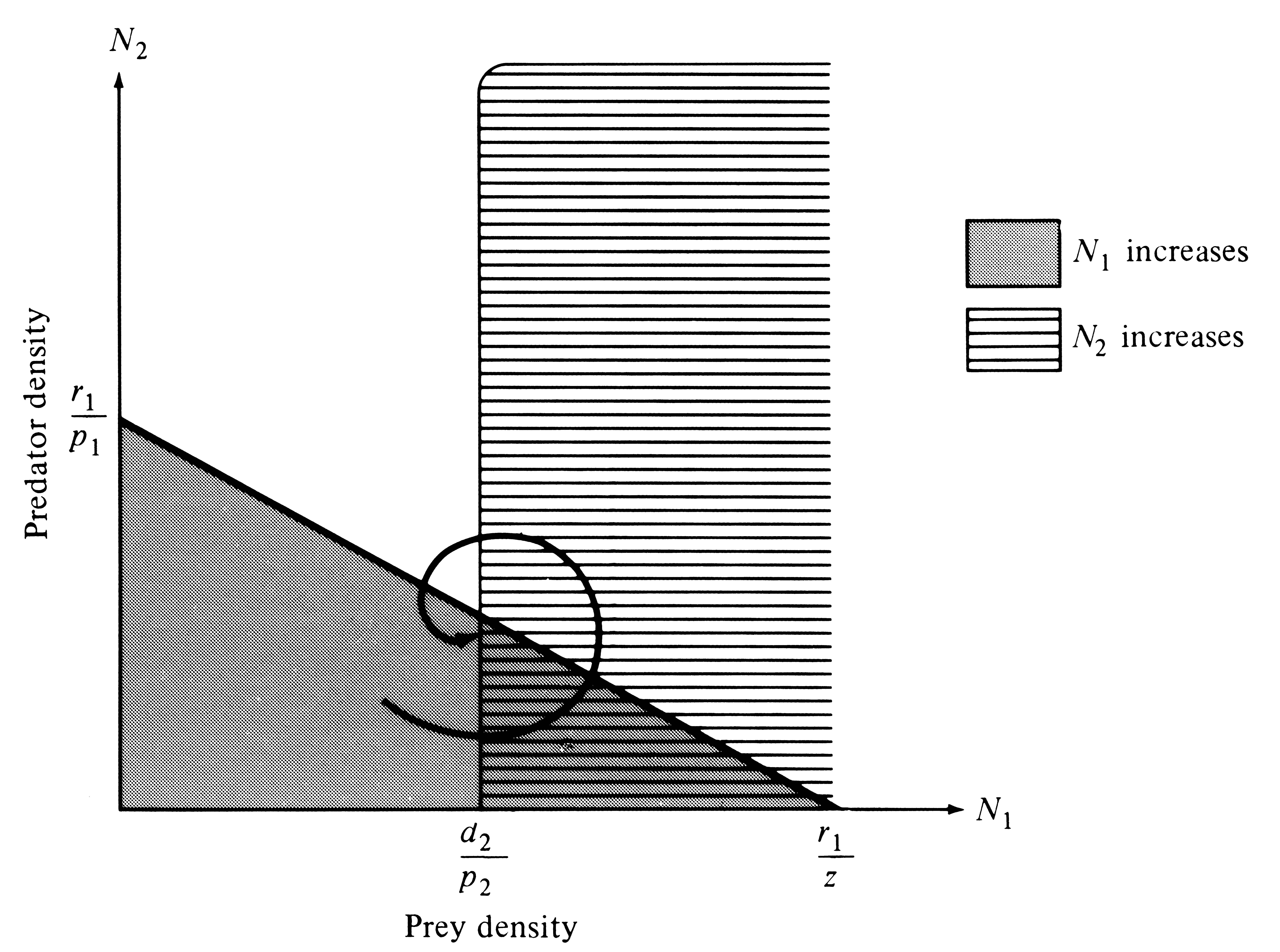
Figure 15.3. Prey and predator isoclines with self-damping in the prey population. Population densities converge on the stable joint equilibrium.
Addition of a simple self-damping term (zN12) to the prey equation results either in a rapid approach to equilibrium or in damped oscillations, both of which lead eventually to the joint equilibrium (Figure 15.3). However, a self-damping term for the predator should include the prey's density as a determinant of the predator's carrying capacity. Perhaps a more realistic (although mathematically less tractable) pair of simple equations for modeling the prey-predator relationship is:
![]() dN1 = r1N1 - z1N12 - β12 N1N2 dN1 = r1N1 - z1N12 - β12 N1N2 ![]() (3) (3)
![]() dN2/dt = γ21 N1N2 - β2N22 / N1 dN2/dt = γ21 N1N2 - β2N22 / N1 ![]() (4) (4)
The prey equation is the simple Lotka-Volterra competition equation, but the predator equation has a new twist in that competitive inhibition of the predator population is now a function of the relative densities of predator and prey. Thus, inhibition of the predator population increases both with increased predator density and with decreased prey density. Notice also that the predator population cannot increase unless there are some prey. However, even though this pair of equations overcome some of the faults of previous pairs, they are still unrealistic in at least one important way. Imagine a situation in which there are more prey than the predator population can possibly exploit; in such a case, growth rate of the predator cannot be simply proportional to the product of the two densities as in equation (4), but some sort of threshold effect must be taken into account.
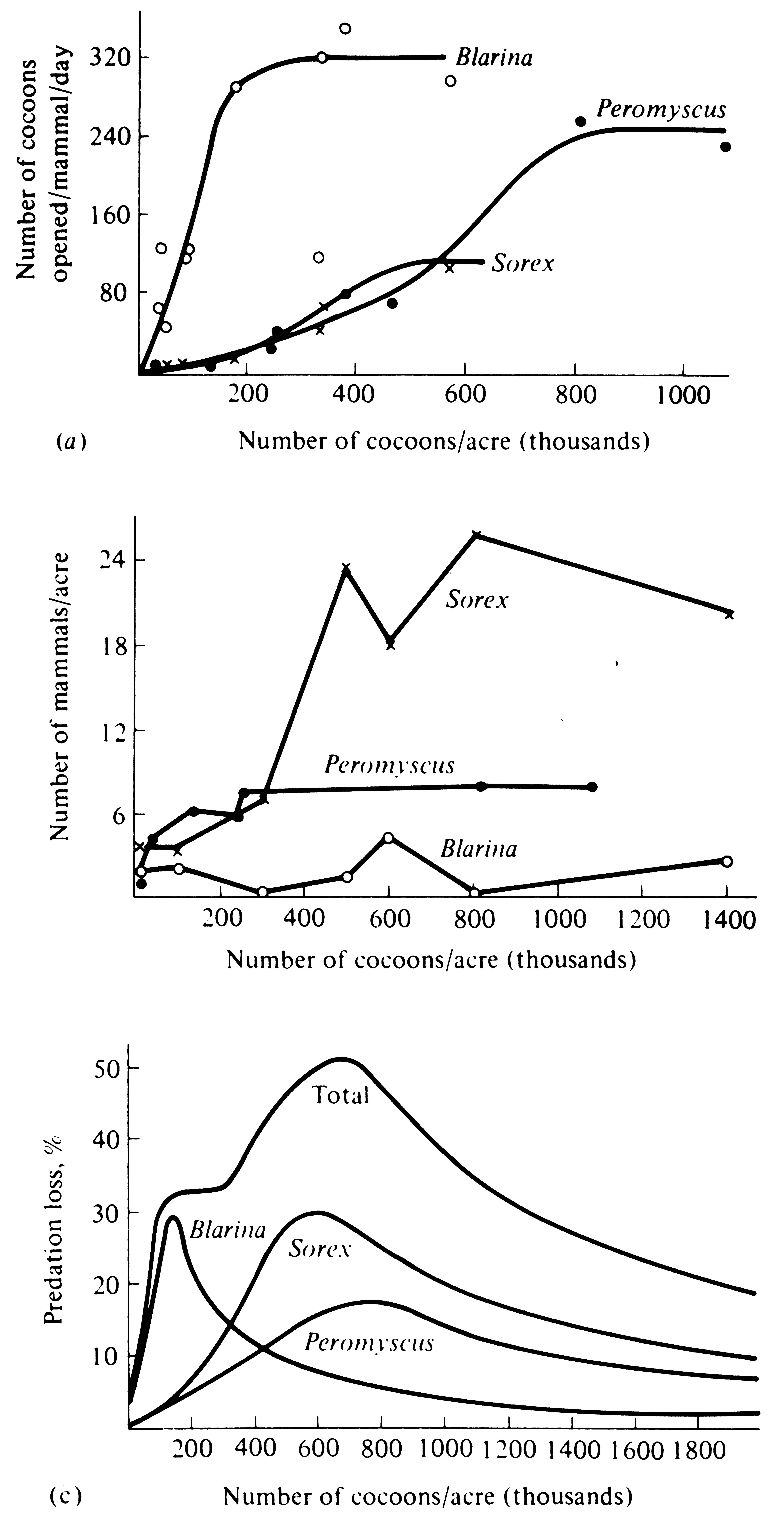
Equations like the preceding ones entirely omit many important subtleties from the prey-predator interaction. For instance, Solomon (1949) distinguished two separate components of the way in which predators respond to changes in prey density. First, individual predators capture and eat more prey per unit time as prey density increases until some satiation threshold is reached, above which the number of prey taken per predator is more or less constant (Figures 15.4 and 15.5a); second, increased prey density raises the predator's population size and a greater number of predators eat an increased number of prey (Figure 15.5b). Solomon termed the former the functional response and the latter the numerical response of the predator.
-
![]() Figure 15.4. Three types of functional responses. [After Holling (1959a).] Figure 15.4. Three types of functional responses. [After Holling (1959a).]
Three types of functional responses are recognized, representing pure forms among a continuum of possibilities (Figure 15.4). [Equations (1) and (3) model a Type 1 linear functional response without a ceiling.] Note that a predator's functional response can allow regulation of prey density without an increase in predator numbers (no numerical response). Using the "systems" approach that relies on continued feedback between observation and model, Holling (1959a, b, 1966) developed elaborate models of predation incorporating both the functional and the numerical responses as well as other parameters, including various time lags and hunger level. These models are more realistic and descriptive than the others (see previous discussion), but they are also more complex and restricted. Obviously a realistic model of prey-predator relationships must be quite complex!
A simple graphical model of the prey-predator interaction was developed by Rosenzweig and MacArthur (1963), who reasoned somewhat as follows. In the absence of predators, the maximum equilibrium population density of the prey is K1, the prey's carrying capacity. Similarly, some lower limit on prey density is likely to exist, below which contacts between individuals are too rare to ensure reproduction and the prey population thus decreases to extinction. Likewise, at any given density of prey, there must be some maximal predator density that can just be supported without either an increase or a
-
Figure 15.5. (a) Number of cocoons (prey) eaten per mammal per day by three small mammals plotted against the density of their prey (the so-called functional response). (b) Density of each of the three mammal predators plotted against prey density (the so-called numerical response of the predators). (c) Combined functional plus numerical responses of each predator species and the total, which represents the overall intensity of predation on the prey population, as a function of prey density. [From Holling (1959a).]
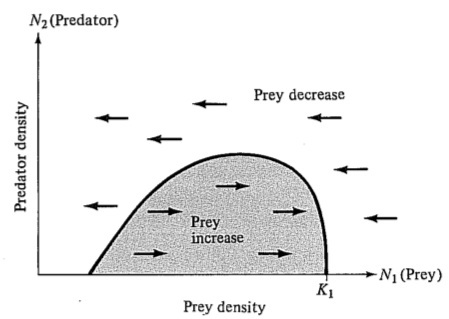
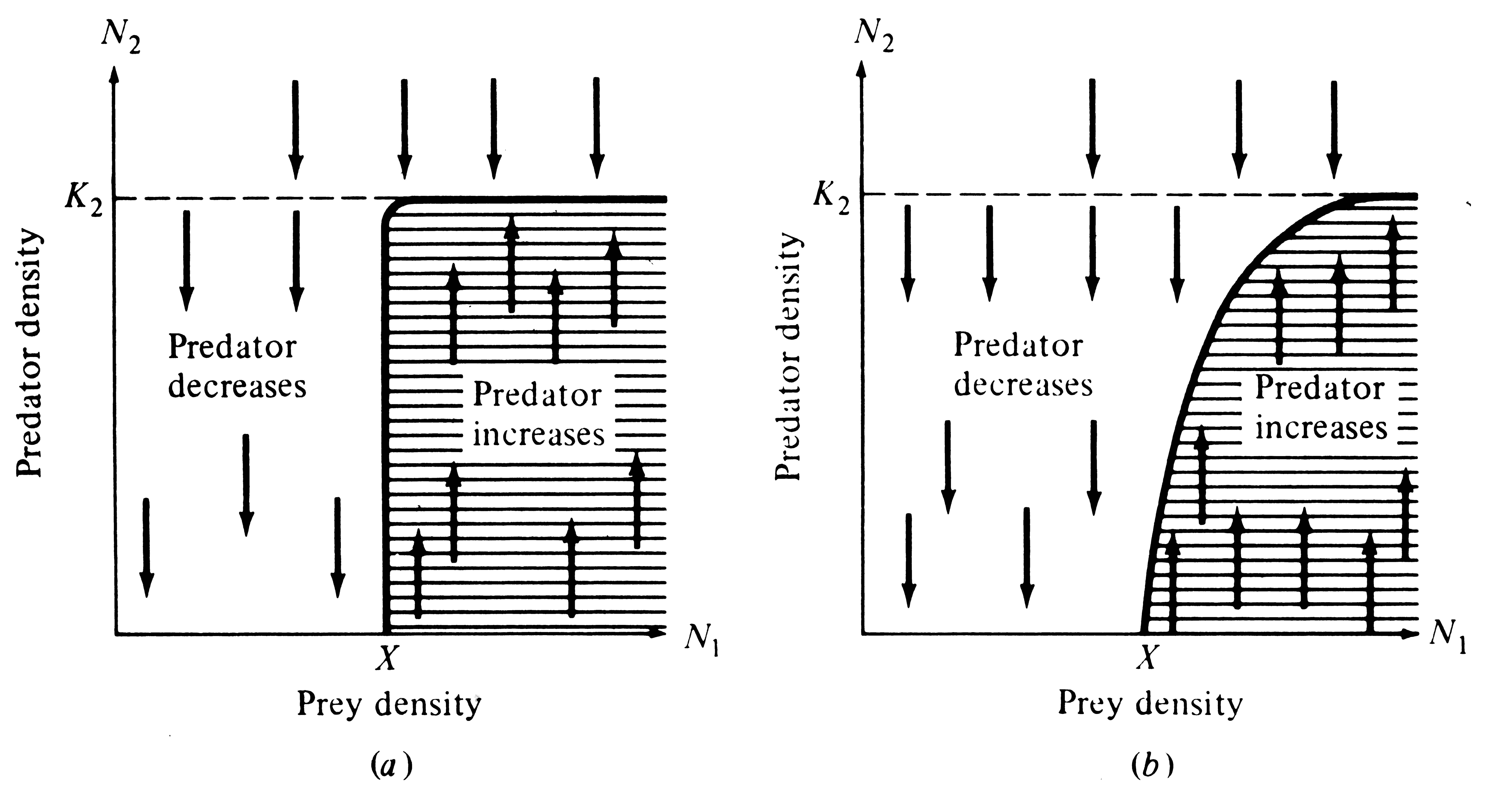
decrease in the prey population. Using these arguments, a prey isocline (dN1/dt = 0) can be drawn in the N1-N2 plane (Figure 15.6) similar to those drawn earlier in Figures 12.3 and 12.4. As long as the prey isocline has but a single peak, the exact shape of the curve is not important to the conclusions that can be derived from the model. Above this line, prey populations decrease; below it they increase. Next, consider the shape of the predator isocline (dN2/dt = 0). Below some threshold prey density, individual predators cannot gather enough food to replace themselves and the predator population must decrease; above this threshold prey density, predators will increase. For simplicity, first assume (this assumption is relaxed later) that there is little interaction or competition between predators, as would occur when predators are limited by some factor other than availability of prey. Given this assumption, the predator isocline should look somewhat like that shown in Figure 15.7a. If there is competition between predators, higher predator densities will require denser prey populations for maintenance and the predator isocline will slope somewhat as in Figure 15.7b. In both examples, the carrying capacity of the predator is assumed to be set by something other than prey density.
-
Figure 15.6. Hypothetical form of the isocline of a prey species (dN1/dt = 0) plotted against densities of prey and predator. Prey populations increase within the shaded region and decrease above the line enclosing it. Prey at intermediate densities have a higher turnover rate and will support a higher density of predators without decreasing.
-
Figure 15.7. Two hypothetical predator isoclines. (a) Below some threshold prey density, X, individual predators cannot capture enough prey per unit time to replace themselves. To the left of this threshold prey density, predator populations decrease; to the right of it, they increase provided that the predators are below their own carrying capacity, K2 (i.e., within the cross-hatched area). So long as predators do not interfere with one another's efficiency of prey capture, the predator isocline rises vertically to the predator's carrying capacity, as shown in (a). (b) Should competition between predators reduce their foraging efficiency at higher predator densities, the predator isocline might slope somewhat like the curve shown. More rapid learning of predator escape tactics by prey through increased numbers of encounters with predators would have a similar effect.
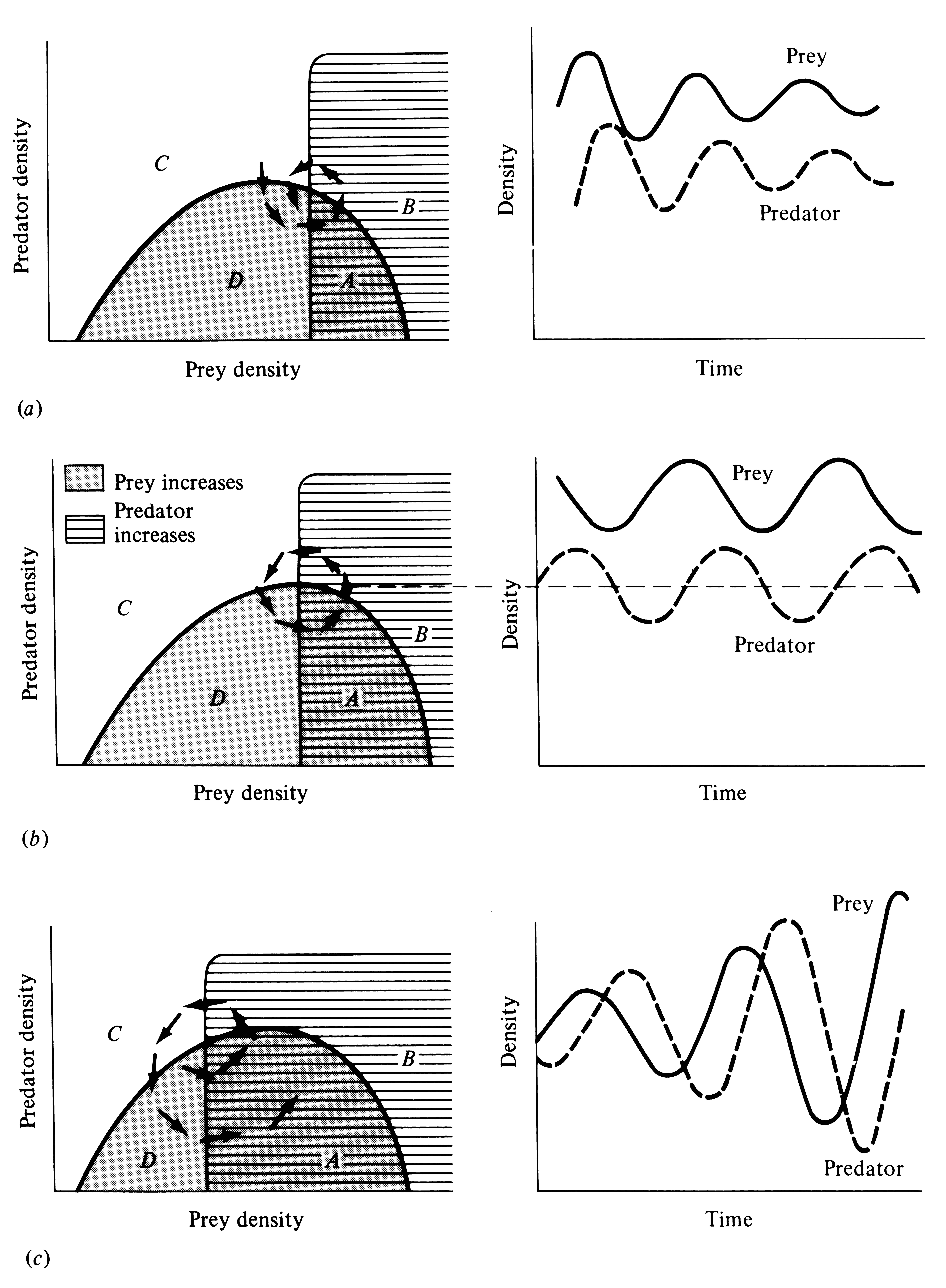
Only one point in the N1-N2 plane represents a stable equilibrium for both species -- the point of intersection of the two isoclines (where dN1/dt and dN2/dt are both zero). Consider now the behavior of the two populations in each of the four quadrants marked A, B, C, and D in Figure 15.8. In quadrant A, both species are increasing; in B, the predator increases and the prey decreases; in C, both species decrease; and in D, the prey increases while the predator decreases. Arrows or vectors in Figure 15.8 depict these changes in population densities.
-
Figure 15.8. Prey and predator isoclines superimposed upon one another to show stability relationships. (a) An inefficient predator that cannot successfully exploit its prey until the prey population is near its carrying capacity. Vectors spiral inward, prey-predator population oscillations are damped, and the system moves to its joint stable equilibrium point (where the two isoclines cross). (b) A moderately efficient predator that can begin to exploit its prey at some intermediate density. Vectors here form a closed ellipse, and populations of prey and predator oscillate in time with neutral stability, as in Figure 15.2. (c) An extremely efficient predator that can exploit very sparse prey populations near their limiting rareness. Vectors now spiral outward and the amplitude of population oscillations increases steadily until a limit cycle is reached, often leading to the extinction of either the predator or both the prey and the predator. Such a cyclical interaction can be stabilized by providing the prey with a refuge from predators. [After MacArthur and Connell (1966).]
Relative magnitudes of the changes in the population densities of prey and predator determine another important property of this model -- that is, whether or not a stable equilibrium exists. There are three cases, corresponding to vectors that (1) spiral inward, (2) form a closed circle, or (3) spiral outward (Figure 15.8a, b, c). These three cases correspond to damped oscillations, oscillations of neutral stability, and oscillations
increasing in amplitude until a limit cycle is reached, respectively (Figure 15.8a, b, c). Such oscillations of prey and predator could perhaps produce population "cycles" like those of lemmings and their predators (later in this Chapter). Given time, the case with damped oscillations will reach its equilibrium value at which neither prey nor predator population densities change; this case corresponds to a predator that is relatively inefficient at gathering prey (the predator cannot even begin to exploit the prey population until prey are fairly near their own carrying capacity). Similarly, the case that produces oscillations of increasing amplitude corresponds to a very efficient predator, which can exploit the prey population nearly down to its limiting rareness. Such an overly efficient predator should rapidly exterminate its prey (and thus, go extinct itself unless alternative prey are available); little wonder that increasing oscillations are never observed in nature! Because of predator escape tactics of prey, many (or most) real predators are probably relatively inefficient, tending to crop only those prey present in excess of a substantial prey population (Errington 1946). Damped oscillations also result from competition among predators, producing a sloping predator isocline (Figure 15.7b). Hence, the case with damped oscillation is probably the most realistic reflection of nature.
Individual predators that reproduce successfully at low prey densities will normally outcompete and replace less efficient individuals that require higher prey densities; hence, natural selection acting on the predator moves its isocline to the left and reduces the stability of the interacting system. However, selection operating in favor of those prey individuals best able to escape predators opposes the action of selection on the predators and forces the predator isocline to the right (presumably it also raises the prey isocline); thus, natural selection on the prey population tends to increase the stability of the system. Indeed, unless the prey is one step ahead of the predator, the latter can be expected to overeat its prey and take both populations to extinction.
"Prudent" Predation and Optimal Yield
Some have suggested that an intelligent predator should crop its prey so as to maximize the prey's turnover rate and therefore the predator's yield. Such a "prudent" predator would maintain the prey population at the density that gives the maximum rate of production of new prey biomass. In terms of the prey isoclines depicted in Figure 15.8, this prey density of "optimal yield" corresponds to the density at the peak of the prey isocline. Humans have the capacity to be such a prudent predator; indeed, optimal yield has long been a goal in management of exploited populations in fisheries biology. But do other, less intelligent predators also maximize their yield? A truly "prudent" predator should prey preferentially upon those prey individuals with low growth rates and low reproductive values and leave those with rapid growth rates and higher reproductive values alone. In fact, predators often do take the aging and decrepit prey individuals, which are frequently easy to catch, while younger, more vigorous ones escape.
However, there is a potential flaw in the prudent predation interpretation, provided that several predator individuals encounter the same prey items. lf, say, young juicy prey are less experienced and easier to catch, an individual predator who "cheated" and ate them would be likely to leave more genes than the prudent genotypes that did not exploit this food supply; as a result, nonprudence would become incorporated into the gene pool and spread. Exactly the same considerations apply to a competing species that is able to use the prey individuals in question. Hence, we would expect prudence to evolve only in a situation where a single predator has exclusive use of a prey population; perhaps some feeding territories are examples.
Another, much more likely, explanation for the occurrence of apparent "prudent" predation in nature can be made in terms of the prey organisms themselves. As was pointed out earlier, the intensity of natural selection is directly proportional to expectation of future offspring (reproductive value); thus, one might predict that individual prey with high reproductive values would have more to gain from escaping predators than would those with lower reproductive values. After one's expectation of future offspring has dropped to zero, nothing further can be gained from being able to escape a predator and predator avoidance cannot be evolved. Thus, many cases of apparent "prudent" predation may well be simply part and parcel of old age; the evolution of senescence has wide significance! Viewed in this way, the susceptibility of prey to predation should be inversely related to their reproductive value.
Selected Experiments and Observations
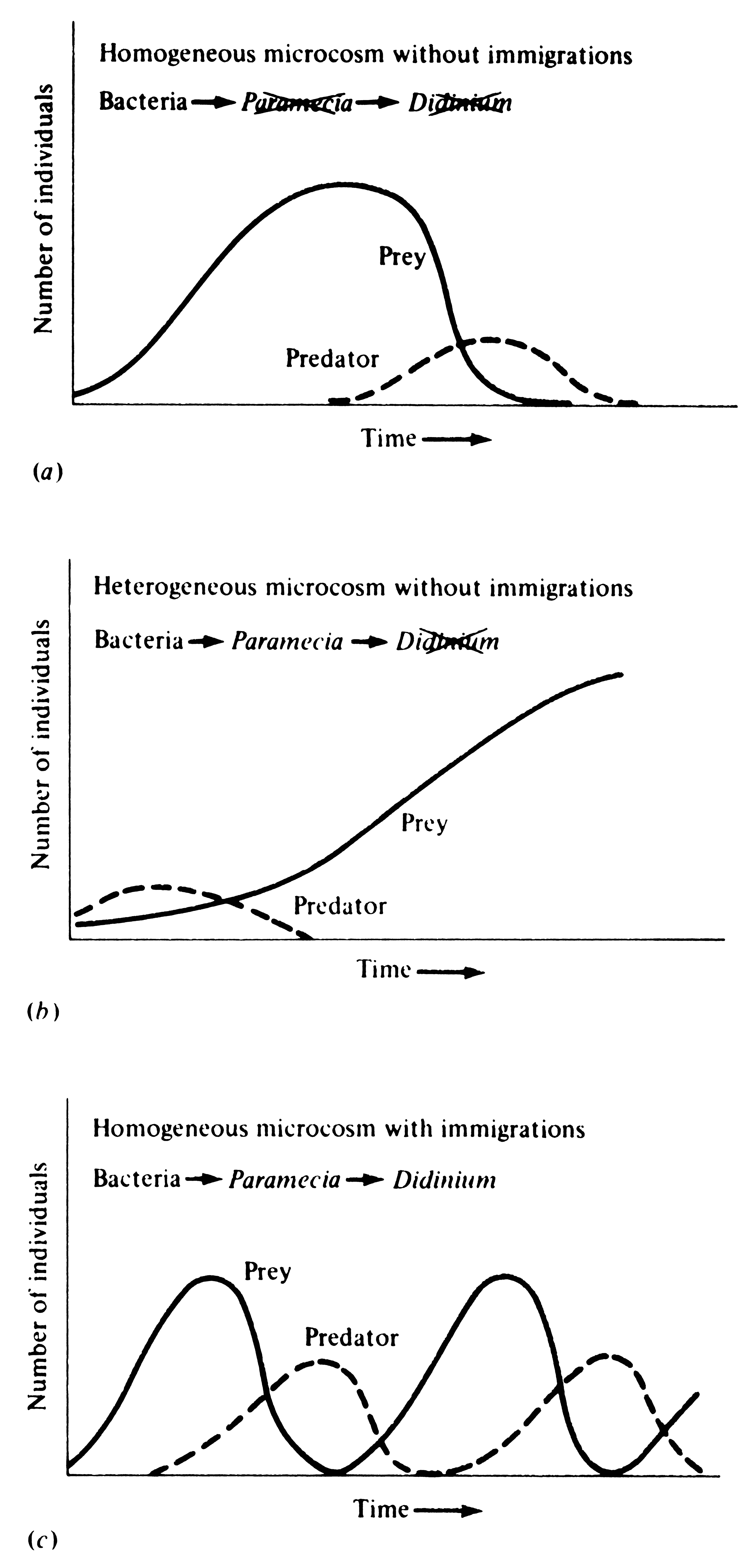
Predation is readily studied in the laboratory and, under certain favorable circumstances, in the field. Gause (1934) studied a simple prey-predator system in the laboratory using two microscopic ciliated protozoans, Paramecium caudatum and Didinium nasutum. Didinium are voracious predators on Paramecium. When both species were placed together in a test tube containing clear medium (which supports a culture of bacteria, the food for Paramecium), Didinium overate its food supply, exterminated it, and then starved to death itself (Figure 15.9a). When some sediment was added to the medium (making it "heterogeneous" rather than "homogeneous"), providing a refuge or "safe site" for the prey, Didinium went extinct but the Paramecium population recovered (Figure 15.9b). In a third experiment (Figure 15.9c), Gause introduced new individuals of each species at regular time intervals; such "immigrations" resulted in two complete cycles of prey and predator. In other experiments, using Paramecium aurelia as the predator and a yeast, Saccharomyces exiguus, as prey, Gause (1935) obtained nearly three complete cycles.
-
Figure 15.9. Three laboratory prey-predator experiments with protozoans. (a) In a simple homogeneous microcosm without immigration of new prey or predators, the predator quickly overeats and exterminates its prey and then all predators themselves starve to death. (b) In a more heterogeneous system, the predator goes extinct and the prey population recovers to expand to its carrying capacity. (c) Even in a homogeneous microcosm, immigration of prey and predators results in both populations oscillating in time. [From Gause (1934), reprint ed. Copyright © 1964 by The Williams & Wilkins Co., Baltimore, Md.]
In an experiment like Gause's with Paramecium and Didinium (but with P. aurelia as prey), Luckinbill (1973) showed that an unstable prey-predator interaction could be stabilized simply by adding methyl cellulose to the medium: this slowed down movements of both prey and predator and reduced the rate of contact between them.
Huffaker (1958) performed similar laboratory experiments on two species of mites, using oranges as the plant food for the system. One mite was herbivorous, eating the oranges; the second mite was a predator on the herbivorous one. In simple systems with oranges close together and evenly spaced, the predator simply overate its prey and both species became extinct. Increasing distances between oranges only lengthened the time required for extinction but did not allow coexistence. However, by introducing barriers to dispersal and making the system still more complex, Huffaker obtained three complete prey-predator cycles. Thus, environmental heterogeneity increased the stability of the system of a predator and its prey. In addition, these experiments illustrate the existence of prey-predator oscillations predicted by theory.
In a more complex laboratory investigation, Utida (1957) examined both competition and predation simultaneously. His system was composed of three species: a beetle (Callosobruchus chinensis) as prey and two species of predatory wasps as competitors (Neocatolaccus mamezophagus and Heterospilus prosopidis). The prey beetle was provided with a continually renewed food supply. Both species of wasps, which have similar life histories, were dependent on the beetle population as a common food source. Population densities of the three species fluctuated widely and erratically, but after four years, some 70 generations later, all three were still coexisting. Populations of the two predatory wasps tended to fluctuate out of phase with one another. Analysis showed that Heterospilus was more efficient at finding and exploiting the beetle population when it was at low densities but Neocatolaccus was more efficient at high prey densities; thus, the competitive advantage shifted between the two wasps as the density of beetle prey changed in time. Utida (1957) thought that fluctuations of the beetle population were caused both by the effects of the two wasp predators as well as by density-dependent changes in the rate of reproduction of the beetle itself. Thus, this system provides an example of coexistence of two competitors on a single resource due to changing abundance of that resource. The stability of the system is apparently a result of biotic interactions.
Evolutionary Consequences: Prey Escape Tactics
Generalized predators feeding on a variety of prey obviously must be adapted to cope with a wider variety of predator escape tactics than more specialized predators that normally deal with fewer types of prey. By diverging in their "strategies" of predator avoidance over evolutionary time, individuals of two (or more) prey species can make it increasingly difficult for a single predator to capture and exploit both prey types efficiently; thus, prey evolution can "force" a predator to restrict the range of foods it eats. Such evolution of a diversity of escape tactics among prey, especially morphological diversity, has been termed aspect diversity (Rand 1967; Ricklefs and O'Rourke 1975; Schall and Pianka 1980).
Antipredator devices are extremely varied; some mechanisms for predator escape are quite simple and straightforward, but others may be exceedingly intricate and subtle. As an example of the former, many lizards dig special escape tunnels in their burrow systems that come up near the surface and allow the lizard to break out should a predator corner it underground.
Behavior and anatomy often make animals difficult to detect and/or to follow; such cryptic adaptations can involve sound, smell, color, pattern, form, posture, and/or movement. Concealing or cryptic coloration is widespread and often depends on appropriate behavior; to hide itself, an animal must select the proper background and orient itself correctly. Some moths normally align themselves with the dark markings on their wings parallel to cracks and crevices of the tree bark substratum. Nearly all diurnal animals and some nocturnal ones are countershaded, with their dorsal (upper) parts darker than their ventral (lower) parts. Lighting from above casts shadows below; in a countershaded animal these balance dorsoventrally, reducing contrast and producing a neutral density -- the net effect, of course, is to make the animal more difficult to see. Countershading occurs in most insects, fish, amphibians, lizards, snakes, birds, and many mammals. A counterexample that proves the point is provided by a few animals that are normally belly up in nature, such as the "upside down" catfish Synodontis nigriventris and certain moth larvae; these animals are darker ventrally than they are dorsally! Because a successful predator must also be inconspicuous in order to catch its prey, crypticity is equally as important to predators as it is to prey.
Many insects resemble parts of plants on which they live, especially leaves, twigs, thorns, or bark; leaf butterflies and walking sticks are familiar examples. Both a green and a brown color phase often occur in such cryptically shaped animals. For example, females of two southern grasshoppers, Syrbula admirabilis and Chortophaga viridifasciata, have green and brown color phases (strangely, males are almost always brown). Green females predominate in wetter, greener habitats; but in immediately adjacent drier and browner areas the brown form is most prevalent (Otte and Williams 1972). Determination of a female's color is not under strict genetic control but is developmentally flexible in response to local conditions.
Actual demonstrations that coloration differences and background color matching have selective value are unfortunately rather scarce. The best documented example is that of the moth, Biston betularia, in England. This moth, along with several hundred other species, has evolved rapidly during the last century in response to human modification of its habitat. In the 1800s, Biston were pale colored, spending their daytime hours on pale, lichen-covered tree trunks. However, with the buildup of industry and concomitant air pollution, the lichens have died and tree trunks in some areas have been covered with a layer of soot and grime, becoming quite dark. In early collections, black moths (melanics) were very rare, but they have become increasingly more common until now these melanistic varieties comprise the vast majority of moth populations in polluted areas. This phenomenon of directional selection, termed industrial melanism, has also taken place in the United States and in Europe. In an elegant series of experiments, Kettlewell (1956) made reciprocal transfers of pale moths from a nonpolluted woods with melanic moths from a polluted area (Table 15.1). These moths, along with resident moths occurring at each locality, were marked with a tiny inconspicuous paint spot beneath their wings, and attempts were made to recapture them on later days. As expected, pale moths had lower survivorship in the polluted woods and melanic moths had lower survivorship in clean, lichen-covered forests. Moreover, Kettlewell actually observed foraging birds catching mismatched moths.
Table 15.1 Numbers of Typical and Melanic Marked Moths (Biston betularia) Released and
Recaptured in a Polluted Woods Near Birmingham and an Unpolluted Woods Near Dorset*
_______________________________________________________________________
![]() Polluted Woods Polluted Woods![]() Unpolluted Woods Unpolluted Woods
_______________________________________________________________________
Numbers of marked moths released
![]() Typical Typical ![]() 64 64 ![]() 496 496
![]() Melanic Melanic ![]() 154 154![]() 473 473
Number of moths recaptured
![]() Typical Typical![]() 16 (25%) 16 (25%)![]() 62 (12.5%) 62 (12.5%)
![]() Melanic Melanic![]() 82 (53%) 82 (53%)![]() 30 (6.3%) 30 (6.3%)
_______________________________________________________________________
* The wild population in the polluted woods was 87% melanic.
Source: From data of Kettlewell (1956).
A black lava flow on white desert sands in New Mexico provides a "natural" experiment that strongly suggests that background color matching has evolved and is adaptive (Benson 1933). This lava flow is completely surrounded by white sandy areas and has presumably been stocked mostly with animals derived from those that live on the white sands. Two closely related pocket mice live in the area: Perognathus intermedius ater is nearly pitch black and occurs only on the lava; P. apache gypsi is pale white and lives only on the white sands.
Some animals are covered with blotches of different colors, which tend to break up their shape and to make them more difficult to see. Good examples are rattlesnakes and boa constrictors; often, these snakes look so much like a pile of dead leaves that they may go undetected. This type of coloration, especially prevalent in larger animals that have difficulty in finding hiding places, such as leopards, tigers, and giraffe, has been termed disruptive coloration.
A form of adaptive coloration that is not necessarily cryptic is called flash coloration. Many inconspicuous insects, including some butterflies and grasshoppers, are extremely cryptic when at rest; but when disturbed they fly away and reveal brightly colored underwings (often red, yellow, or orange). These insects thus suddenly become extremely visible and conspicuous, catching the predator's eye. When they land, they close their wings and quickly move away from the spot at which they landed. As anyone who has chased grasshoppers knows, it is very difficult to keep track of the position of such an animal. (Squid and octopi employ a remotely similar strategy when they squirt out their "ink," leaving a dense cloud in the water: Typically, the animal immediately changes both color and course, becoming pale and swimming at right angles to its original direction of flight, thereby evading the predator.) A rather derived kind of flash coloration occurs in some butterflies and moths that have large owl-like eyes on their underwings. These eyes are normally hidden by the upper pair of wings. When a small bird approaches, the upper wings are suddenly twitched aside, revealing an owl-like face beneath. Some small birds are apparently so startled that they fly away, leaving the insect alone! Some such eyespots are perpetually on display (Stradling 1976). Similarly, the yellow "eyes" on some large green caterpillars may make them resemble green tree snakes. Certain tropical butterflies have even evolved remarkably realistic snake head scalation patterns on the undersides of their wings.
Another, smaller type of eyespot actually invites a predator's attack. Many predators instinctively go for the eyes of their prey because eyes are usually one of the most vulnerable parts of an animal and the loss of vision readily incapacitates prey (lions and wolves have found another "Achilles heel" on large ungulates -- they simply hamstring the animal). Many species of butterflies possess small "fake" eyespots along the periphery of their wings that may actually invite attack. Eyespots painted on such butterflies in places without eyespots are damaged when the animals are released and recaptured, apparently from being pecked at by birds (Sheppard 1959). Thus, the butterfly obtains a second chance at escape by luring the predator's attack away from its own eyes. Behavioral adaptations may sometimes serve similar functions; certain snakes raise their blunt tails and wave them around in a very headlike manner, occasionally actually making short menacing lunges with their tails at the threatening predator! Should the unwary predator grab the snake by this "head," the serpent still has its real head free to bite back.
Many birds and mammals have various sorts of alarm signals, that, when given, warn other animals that a predator is in the immediate vicinity. Beaver warn one another of danger by slapping their tails loudly against the surface of the water. Similarly, rabbits in a warren (a colony of related individuals using the same burrow system) often "thump" with their hind feet to signal the approach of a predator. The white underside of the tail of some rabbits and ungulates (such as the white-tailed deer) is raised when the animal flees from a predator, perhaps serving as a warning signal to nearby animals. Prairie dogs, many primates, and many foraging bird flocks, such as crows, frequently post sentinels that watch for predators from a good vantage point and warn the group should one appear in the distance. Among birds, alarm calls in response to the presence of bird-eating hawks are especially prevalent. Typically, these faint shrill whistles are extremely difficult for a vertebrate predator to locate; often, they are similar in widely different bird species, presumably having converged over evolutionary time.
Because different species may benefit from the call of one individual, hawk alarm calls appear to be somewhat altruistic. However, warning calls are normally used only during the nesting season and are therefore best interpreted as having arisen through kin selection. The ventriloquial nature of the call, coupled with the fact that the caller has already seen the predator and therefore knows exactly where the danger lies, ensures that the risk to the bird giving the alarm is slight. Likewise, the fact that banded birds often return to breed in the same area where they themselves were raised means that birds in any given area will be related and share many genes, which in turn ensures that the total gain to the many relatives of the pseudoaltruist will frequently be quite large. If hawk alarm calls have evolved via kin selection, one would predict that the frequency of use of such calls should be inversely related to the distance the caller is from its own nest and immediate relatives (no one has yet demonstrated this empirically). The fact that these calls work across species lines is easily explained because individuals that recognize warning signals of other sympatric species as alarms should be at a relative advantage within their own population. The convergence of hawk alarm calls can be explained by either or both of two mechanisms. The first concerns the ventriloquial properties of the call. The number of ways in which an alarm call can be loud enough to function as a warning, and yet still be difficult to locate, are decidedly limited. Thus, convergence could be a simple result of physical constraints inherent in the system. A second, equally likely, possibility is that natural selection has favored convergence because it facilitates interspecific recognition of alarm calls. Thus, individuals tending to produce call variants more like those of another species would benefit because they would also be more likely to recognize the calls of the other species (as such, call convergence would be very similar to Müllerian mimicry, discussed later).
Several alternative explanations for alarm calls exist. The calls may not be intended as "alarms" at all, but as signals intended to inform the predator that it has been detected and that it need not try to capture the caller. Charnov and Krebs (1975) suggest that the caller could decrease its own chance of being captured by alerting other individuals because the predator's attention would be drawn away from the caller and toward the large numbers of others scurrying for cover. Still another argument for a selfish caller is based on the fact that many predators tend to stay nearby and return to an area where they have successfully captured prey in the past; to the extent that alarm calls prevent a predator from catching any prey at all, they may serve to keep the predator on the move and hence out of the immediate area of the caller. Some birds that forage in mixed-species flocks in the tropics issue false alarm calls when they see a juicy prey item, which distracts the attention of other birds, allowing the selfish caller to capture the prey item (Munn 1986).
Yet another evolutionary consequence of predation is warning coloration; unpalatable or poisonous animals have often evolved bright colors that advertise their distastefulness. Such markings are called aposematic, which translates as "away signal." These animals are usually colored with the same conspicuous colors we use for signs along highways: reds, yellows, and blacks and whites. Examples of animals with warning coloration are bees and wasps, monarch butterflies, coral snakes, skunks, and certain brightly colored poisonous frogs and salamanders. Signals that warn potential predators may also involve pattern, posture, smell, or sound. A rattlesnake's buzzing presumably serves to warn other larger animals such as the American bison not to come too near (unfortunately for the rattler, however, this warning only attracts human attention, which usually results in the snake's demise). Experiments have shown that avian and lizard predators learn to avoid distasteful prey. In the case of poisonous prey, this learning may actually become incorporated into the gene pool and manifested as an "instinctive" avoidance.
Mimicry is an interesting sidelight of warning coloration that nicely demonstrates the power of natural selection. An organism that commonly occurs in a community along with a poisonous or distasteful species can benefit from a resemblance to the warningly colored species, even though the "mimic" itself is nonpoisonous and/or quite palatable. False warning coloration is termed Batesian mimicry after its discoverer. Many species of harmless snakes mimic poisonous snakes; in Central America some harmless snakes are so similar to poisonous coral snakes that only an expert can distinguish the mimic from the "model." Similarly, harmless flies and clearwing moths often mimic bees and wasps, and palatable species of butterflies mimic distasteful species. Batesian mimicry is disadvantageous to the model because some predators will encounter palatable or harmless mimics and thereby take longer to learn to avoid the model. The greater the proportion of mimics to models, the longer is the time required for predator learning and the greater the number of model casualties. In fact, if mimics became more abundant than models, predators might not learn to avoid the prey item at all but might actively search out model and mimic alike. For this reason Batesian mimics are usually much less abundant than their models; also, these mimics are frequently polymorphic and mimic several different model species.
A different kind of mimicry occurs when two species, both distasteful or dangerous, mimic one another; this phenomenon is termed Müllerian mimicry. Both bees and wasps, for example, are usually banded with yellows and blacks. Because potential predators encounter several species of mimics more frequently than a single species, they learn to avoid them faster, and the relationship is actually beneficial to both prey species (Benson 1972). The resemblance need not be so close as it must be under Batesian mimicry because neither species actually deceives the predator; rather, each only reminds the predator of its dangerous or distasteful properties. Müllerian mimicry is beneficial to all parties including predators; mimics can be equally common and are rarely polymorphic.
Plants, being sessile, cannot use many of the escape techniques of animals and are obviously and decidedly more limited in the ways they can deter potential predators. A plant with a patchy or spotty distribution in time and/or space may escape some predation simply by virtue of its unpredictable availability; thus, an annual that is here today but gone tomorrow may be more difficult for herbivores to find and use than an evergreen perennial that is always relatively available (see subsequent discussion). Some plants, especially perennials, have evolved morphological adaptations, such as hairs, spines, and hooks (Gilbert 1971), that discourage many herbivores quite directly. By far the most widespread predator deterrent of plants is what might be termed chemical warfare. A great variety of secondary chemical substances occur in plants that are not known to serve any direct physiological function for their possessors. Many are evidently not breakdown products of larger molecules due to metabolic processes and wastes but are secondary substances produced by active synthesis from smaller molecular precursors. Such secondary chemical substances often contain nitrogen and other elements that are available to the plant only in limited supply; moreover, energy is required to produce these chemicals. Clearly, there are definite costs to the plant in production of herbivore repellents. Nearly a century ago the German botanist Stahl (1888) suggested that these secondary substances might reduce the plant's palatability to herbivores. Stahl's prediction has now been amply verified for many different plant-herbivore systems.
Agriculturalists and plant breeders have produced many strains that are highly resistant to normal herbivores. Genetic varieties, or morphs, of plants in nature have been shown to be differentially palatable to herbivores; thus, Jones (1962, 1966) found that a number of herbivores ranging from insects and snails to Microtus (meadow mice) preferred a "noncyanogenic" morph of the plant Lotus corniculatus over a "cyanogenic" one. Tannins, molecules that bind to proteins such as digestive enzymes, have been implicated as the agent that repels some herbivores; oak leaf tannin significantly reduces the growth rate of larvae of the moth Operopthera brumata (Feeny 1968). Frequently, herbivores eat only relatively new growth and do not utilize older parts of plants, presumably because the latter contain tannins and other repellent chemicals (Feeny 1970). Other chemical substances believed to protect plants from animals and fungi include essential oils and resins, alkaloids, terpenes, and terpenoids. The latter two classes of compounds have especially penetrating odors and tastes; sesquiterpenes are fatal to sheep. Wild herbivores that have evolved alongside poisonous plants would be unlikely to eat them, whereas domestic sheep and cattle will eat many poisonous fodders.
The argument presented at the beginning of this section suggests that sympatric plant species may usually gain by evolving qualitatively different antiherbivore secondary chemical defenses. As a result, plants in general are not continuous resources but constitute a spectrum of qualitatively distinct, discrete food types. Such divergent anti-herbivore chemistries force evolution of herbivore specialists by reducing the efficiency of generalized herbivores. Because related plants have similar secondary chemistries, phylogenies of certain herbivorous insects (particularly butterflies) closely parallel the phyletic relationships of their host plants (Ehrlich and Raven 1964; Benson, Brown, and Gilbert 1975).
Parasitism
Parasitism is similar to predation in terms of the signs of the interaction coefficients between the two populations, but parasitism differs from predation in that members of the species affected detrimentally (the "host") are seldom killed outright but may live on for some time after becoming parasitized. Batesian mimicry and herbivory can be considered as special cases of parasitism. So-called parasitoid insects, such as ichneumonid wasps, which lay their eggs in or on another host insect (larvae develop inside the host, consuming and eventually killing it), merge into more traditional predation.
Parasitism has been defined using several criteria (Kennedy 1975): (1) the parasite is physiologically dependent on its host; (2) the parasite usually has a higher reproductive potential than its host (for successful dispersal and infection, parasites typically must be exceedingly fecund); (3) parasites are capable of killing highly infected hosts; (4) the infection process tends to produce an overdispersed distribution of parasites within the host population. In addition, parasites are typically substantially smaller than their hosts and usually have a much shorter generation time than do their hosts. As a result, parasites typically evolve much faster than their host species. A major problem facing most parasites is how to get transmitted from host to host, and parasites have evolved numerous clever mechanisms to accomplish transmission.
Several fundamentally different classes of parasites may be distinguished. Ectoparasites, such as fleas, ticks and mites, exploit the outer surface(s) of their hosts, whereas endoparasites live inside their host organisms (these are often called pathogens or disease agents). A spectrum can be envisioned ranging from microparasites to macroparasites to parasitoids to predators. A wide variety of important ecological characteristics change along this continuum (Table 15.2).
Table 15.2 Comparison of Certain Ecological Characteristics That Vary Along a Parasite-Predator Spectrum
_________________________________________________________________________________________
Characteristic![]() Microparasite Microparasite![]() Macroparasite Macroparasite![]() Parasitoid Parasitoid![]() Predator Predator
_________________________________________________________________________________________
Body size![]() Much smaller Much smaller![]() Smaller than Smaller than![]() Mature stages Mature stages![]() Larger Larger
![]() than hosts than hosts![]() hosts hosts![]() similar in size similar in size![]() than prey than prey
Intrinsic rate![]() Much faster Much faster![]() Faster than Faster than![]() Comparable Comparable![]() Usually Usually
of population![]() than hosts than hosts![]() hosts hosts![]() but slightly but slightly![]() slower slower
growth ![]() slower slower![]() than prey than prey
Interaction with![]() One host One host![]() One host One host![]() One host can One host can![]() Many prey Many prey
host individuals![]() usually supports usually supports![]() supports a few supports a few![]() support several support several![]() items are items are
in natural![]() several populations several populations![]() to many individuals to many individuals![]() individuals of individuals of![]() eaten by each eaten by each
populations![]() of different species of different species![]() ![]() different species different species![]() predator predator
Effect of the![]() Mildly to fairly Mildly to fairly ![]() Variable, not Variable, not![]() Eventually Eventually![]() Usually Usually
interaction on![]() deleterious deleterious![]() too virulent to too virulent to![]() fatal fatal![]() immediately immediately
host individual![]() definitive; can definitive; can ![]() fatal fatal
![]() be intermediate be intermediate
Stability of the ![]() Intermediate Intermediate![]() High High![]() Intermediate Intermediate![]() Usually low Usually low
interaction between
trophic levels
Ability to![]() Moderate Moderate![]() Low Low![]() Fairly high Fairly high![]() High High
regulate the
lower trophic
level
_________________________________________________________________________________________
Sources: Modified from Anderson and May (1982) and Toft (1986).
Another different but interesting type of parasitism is social parasitism, which includes thievery, slavery, and brood parasitism. Best known in birds, brood parasitism occurs when members of one species exploit another for parental care, usually by means of deceiving the hosts into believing that they are raising their own progeny (cowbirds are the most familiar example).
As in predator-prey interactions, parasites and their hosts are typically engaged in antagonistic evolutionary interactions due to the inherent basic conflict of interests. Selection should always favor resistant host individuals (Haldane 1941). To the extent that natural selection favors evolution of reduced parasite virulence (see also subsequent discussion), parasitic interactions may evolve gradually toward commensalisms and ultimately even become mutualistic interactions.
![]() Parasitism ----> Commensalism ----> Mutualism Parasitism ----> Commensalism ----> Mutualism
![]() (+ , -) (+ , -)![]() <----- <-----![]() (+ , 0) (+ , 0) ![]() <----- <----- ![]() (+ , +) (+ , +)
Of course, selection could also proceed in the opposite direction (reverse arrows). Such changes may also occur during ecological time, as during the ontogeny of parasites.
Most parasites are very specialized. Many endoparasites have become intimately dependent on their hosts; tapeworms have lost their digestive systems and viruses consist of little but "naked" genetic material (indeed, they are not really even "alive" outside of their hosts). Elaborate life cycles involving intermediate hosts and various infective stages are prevalent among parasites.
A degree of specificity typically arises in parasite-host interactions, and many parasites have evolved species-specific host requirements. An interesting ramification of the very intimate interaction between parasites and their hosts is "molecular mimicry." In this phenomenon, antigenic determinants of parasitic origin (known as "eclipsed antigens") resemble host antigens to such an extent that they do not elicit the formation of host antibodies. This, of course, allows the parasite to dwell safely inside the host's tissues in a more or less uncontested fashion, protected from the host's immune response. Parasites exploit many other innovative adaptations to confound their hosts; some flagellate protozoans called trypanosomes which cause sleeping sickness simply switch antigens -- every time the host mounts an immune response, the parasite "sheds" its previous coat and puts on another (Turner 1980). Some tapeworms secrete copious amounts of a sticky substance composed of proteins and sugars. Host antibodies get gummed up in this "glycocalyx"; some tapeworms produce such vast amounts of glycocalyx that the host's antibodies can never find the worm. Clearly, this host defense tactic costs tapeworms a substantial amount.
Among the many subtle aspects of parasite biology is host-altered behavior, in which parasites actually cause increased vulnerability of infected hosts to their own parasites and/or predators to facilitate transmission of parasites to new host individuals (Holmes and Bethel 1972; Moore 1985, 1987). A simple example is a rabid animal's biting other animals and thereby passing on the rabies viral infection. (A venereal disease causing increased sexual activity/promiscuity would be similar.)
A somewhat more complex example involves the lancet fluke Dicrocoelium dentriticum, whose primary host is sheep; metacercariae of this trematode infect ants as intermediate hosts. One or two metacercariae encyst in subesophageal ganglia of an ant's brain, which affects the behavior of the ant, causing it to climb to the tip of a grass stem and to clamp its mandibles closed there late in the day. As temperatures fall, the ant's jaws become locked and it cannot move. This behavior, of course, places the infected ant in the position where it is most likely to be inadvertently ingested by a grazing sheep early the next morning.
A prevalent conception among parasitologists is that very virulent parasites represent recently evolved parasite-host interactions, whereas more benign parasites are indicative of older, more ancient associations. Although this assertion can be disputed, evidence exists for the evolution of reduced virulence. One of the best examples concerns a carcinogenic myxoma virus, which produces a mild nonlethal disease in its natural host, the South American cottontail rabbit Sylvilagus brasilensis. In 1950, this virus was used as a biological control agent in Australia in an effort to eradicate the introduced European rabbit Oryctolagus cuniculus. Fleas and mosquitos transmit the virus between infected rabbits. Initially, this virus proved exceedingly virulent in Oryctolagus, causing better than a 99 percent kill among infected rabbits. Of course, selection was intense for increased resistance among rabbits. Even so, a less virulent strain of myxoma virus rapidly became established in the field. Apparently, the exceedingly virulent strain often killed its host outright even before being transmitted to other rabbits, whereas less virulent strains were more likely to persist long enough to be passed on to other hosts. In other situations, such as when a parasite finds itself engaged in a race against its host's immune response, selection may actually favor increased virulence. Influenza spreads from host to host when people are out in public sneezing and coughing -- thus, it is very much in the virus's best interests not to make hosts feel too sick. However, in a vector-borne disease like malaria, virulence incapacitates the host with high fever (heat attracts mosquitos) and actually makes it easier for mosquitos to bite infected people and spread the disease. Thus, natural selection should favor different levels of virulence for parasites with different types of transmission between hosts (Ewald 1994).
Parasites can have rather profound effects on the ecology of their hosts. Effects of the malarial parasite Plasmodium mexicanus on members of a population of Sceloporus occidentalis lizards were examined in California by Schall (1982). About one-third to 40 percent of male lizards were infected, whereas only 15 to 30 percent of female lizards had malaria (percentage infected increases with age). Blood hemoglobin levels were lower in parasitized lizards than in unparasitized ones. When lizards were at rest, oxygen consumption rates did not differ between the two groups, but during maximal activity, infected animals had significantly lower metabolic rates. Moreover, both the capacity for aerobic metabolism and running stamina (as measured in an oval track) were reduced in infected lizards as compared to controls. Parasitized lizards also tended to have smaller fat reserves and smaller clutch sizes than noninfected ones. Effects of infection by malarial parasites might be subtle and hosts might appear healthy, but these parasites seem to reduce host fitness substantially.
In another study, Schall (1992) demonstrated that another species of malarial parasite allowed coexistence of two species of Caribbean Anolis lizards (in the absence of the parasite only one species of lizard occurs, but if this species of lizard is parasitized, the other lizard species can coexist with it).
Like predators, parasites can affect community structure, although sometimes in obscure ways. Recall that the outcome of interspecific competition between two species of flour beetles could be reversed by a protozoan parasite (Park 1948). Avian malaria may have contributed to the extinction of some members of the Hawaiian avifauna.
Epidemiology
Disease transmission lends itself quite naturally to quantitative treatment (Anderson 1981). Among phenomena one can examine are (1) the percentage of hosts that are susceptible, infected, or immune, (2) rate of spread of the pathogen under different conditions (particularly with respect to host density and variation in transmission rates between different subgroups of the population and the frequency of disease introduction [infection]), and (3) the extent to which density-dependent probability of infection regulates host population growth. The stability of the interaction and the evolution of host resistance and disease severity are also of considerable interest.
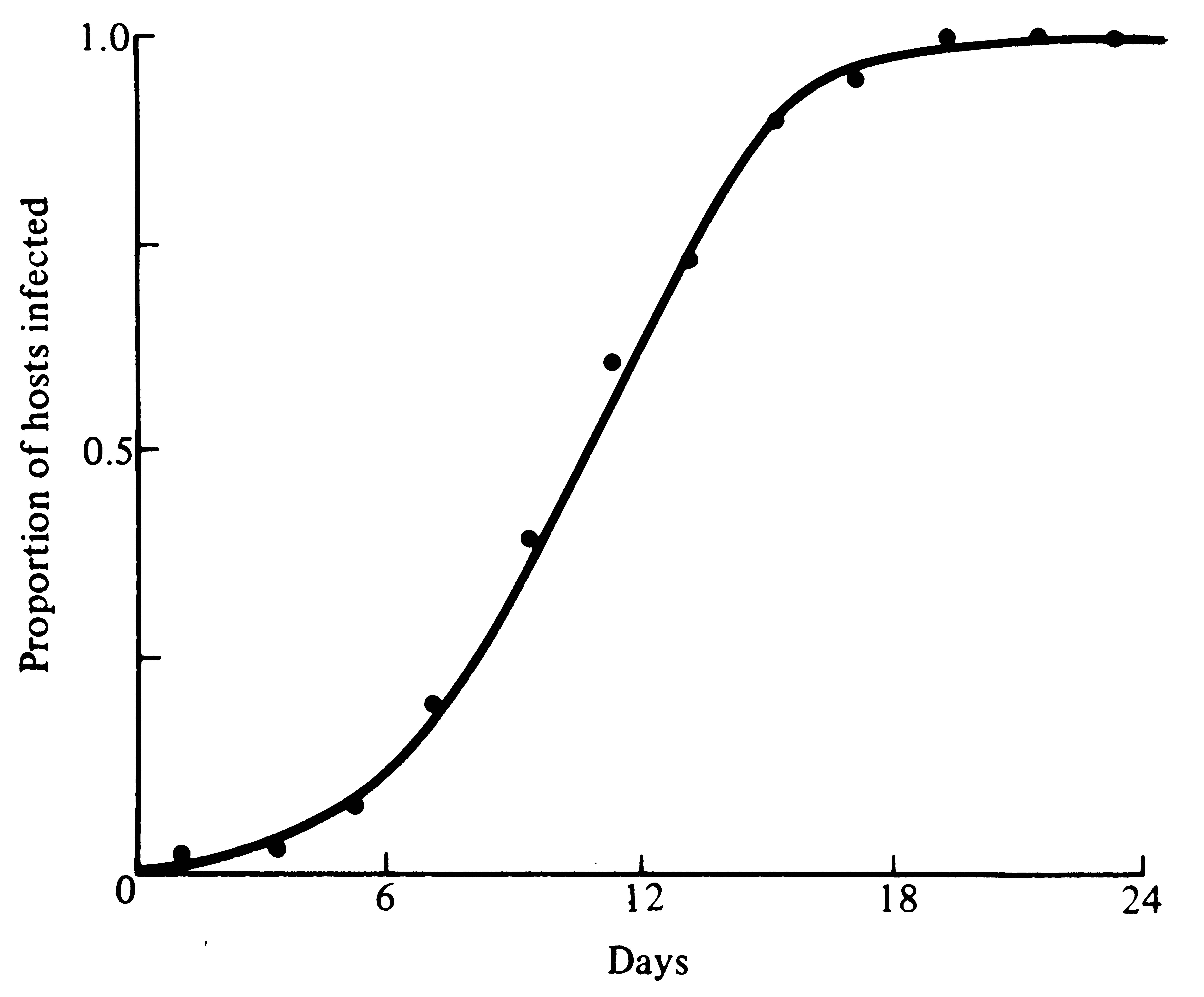
Smallpox epidemics in human populations were modeled mathematically by Bernoulli over two centuries ago. Epidemiological models often make the simplifying assumption that host population size is constant, and examine the dynamics of parasitism, usually in terms of the proportion of hosts infected. Two rate parameters are critical: rate of transmission of the disease from infected to susceptible hosts and the rate at which infected hosts recover to become immune. A critical quantity is the basic reproductive rate of the infection (also called the basic reproductive ratio). Can a single infected individual in an otherwise fully susceptible population produce more than one new infection (leading to an epidemic) or fewer than one? (This is analogous to the net reproductive rate.) In such a simple epidemiological mathematical model, two equilibria exist: one with no infection and the other with constant but dynamically renewing proportions of hosts in each of three states, susceptible, S, infected, I, and immune, R (for recovered). Interestingly, which of these two equilibria exists depends both on the two rate parameters and on what is termed the threshold host population size, or the critical density of hosts necessary for parasites to replace themselves and to spread. In small host populations, parasites cannot infect new hosts rapidly enough to survive, whereas an epidemic may take with the same parameters in a larger host population. Such epidemiological models suggest that vaccination efforts should be more intensive in urban areas than in rural ones (country folks are less likely to get infected than city slickers!). With no lag in transmission and with no recovery, the time course of an epidemic is sigmoidal (Figure 15.10).
-
Figure 15.10. The time course of an epidemic is typically sigmoidal, with the rate of new infection reaching its maximum when about half the population is infected, while the other half is vulnerable.
Such a system can be modeled with sets of simple differential equations:
![]() dI/dt = βIS dI/dt = βIS![]() (5) (5)
![]() dS/dt = - βIS dS/dt = - βIS![]() (6) (6)
where β represents the rate of infection, I is the number of infected host individuals, and S is the number susceptible to the pathogen. With such a linear functional response, the parasite spreads slowly at first and its rate of spread is maximized when half the host population is infected and the other half is vulnerable to infection. A more realistic set of equations that includes some host demography was proposed by Bailey (1957) which take the form:
![]() dS/dt = uN - βIS - uS dS/dt = uN - βIS - uS![]() (7) (7)
![]() dI/dt = βIS - vI - uI dI/dt = βIS - vI - uI![]() (8) (8)
![]() dR/dt = vI - uR dR/dt = vI - uR![]() (9) (9)
where R is the number of hosts in recovery, and N = S + I + R and β is the rate of infection, v is the rate of removal of infected individuals (recovery rate), and u is both the per capita birth and death rate of the host population. For diseases with very fast transmission dynamics, host demography can be ignored (u = 0), but the constant supply of susceptible newborn hosts is usually an essential element of long-term disease persistence (which of course is why the removal of this supply through infant vaccination programs is such an important part of infectious disease control programs).
The basic reproductive rate is given by βN/(v + u). For an infectious disease agent to enter a population of susceptible hosts and spread (one infection will start more than one new infection), its basic reproductive rate must exceed 1. Setting this equation equal to 1 and solving for N gives the critical population size necessary for large epidemics:
![]() N(crit) = (v + u)/β N(crit) = (v + u)/β ![]() (10) (10)
In still more complex models with inoculation, time lags, and immunity, interactions between parasites and their hosts can generate cycles of epidemics, which, of course, are all too familiar to parents and pediatricians.
The combination of our high population density, mobility, and sociality makes humans an ideal epidemiological environment for microbes -- microbes will inevitably continue to evolve that can exploit this enormous resource base. Unless we choose to control our own population sizes, microbes will eventually evolve that will control human populations (perhaps this has already happened with the HIV virus?).
Darwinian Medicine
Until recently, a common medical practice has been to "treat
the symptoms" without asking whether or not symptoms represent host defenses or parasite manipulation.
Physicians distinguish between "signs" and "symptoms" of disease with the former being
objective, and the latter, subjective manifestations of the disease.
Inflammation is a major "cause" of disease
in many infections such as tuberculosis. Fever and inflammation are often viewed
as totally undesirable and medications such as aspirin that reduce both are often prescribed. Studies with
desert iguanas have demonstrated that these lizards select higher body temperatures when they are infected
with parasitic microbes than they do when uninfected. Moreover, if infected lizards are prevented from being
able to attain higher temperatures, the microbes prosper and infected lizards suffer. A recent study
(Brandts et al. 1997) demonstrated that use of drugs to reduce fever prolonged the time required to clear
Plasmodium malarial parasites from the blood of human children in Gabon. Some fevers appear to be
adaptive responses to infection, harming microbes more than their hosts. Similarly, inflammation may often
be an integral part of the healing process. During an influenza infection, iron levels in the blood are reduced,
prompting physicians to prescribe iron additives -- however, low iron levels appear to be a host defense against
microbes, essentially starving them out. Providing supplemental iron could actually assist the microparasites!
Similarly, sugars and vitamin C may aid some cancerous cells.
Microparasites with short generation times evolve rapidly. Many
microbes, such as the HIV virus, actually evolve during the course of infection
within a single host. Resistant microbes have evolved in direct response to contact with antibiotics and
will continue to do so. Overuse of antibiotics selects for resistant strains. When antibiotics are fed to
our food animals to enhance yield, resistant bacteria evolve that are passed on to humans. Genes for
resistance to antibiotics are actually transferred between different species of bacteria via exchange of
plasmids -- in 1976, a strain of gonorrhea got genes from the human intestinal bacterium Escherichia coli
that code for an enzyme which destroys penicillin, instantly creating a powerful new antibiotic-resistant
strain of gonorrhea. Many children now suffer from chronic ear infections (otitis media). The middle ear is
a poorly vascularized anatomical location that is difficult to reach with traditional antibiotics -- by
selecting for resistant microbes, we may have inadvertently caused this malady. Health care authorities
are concerned that we may not be able to treat such antibiotic-resistant strains.
"Darwinian medicine" refers to adopting an evolutionary approach to medical treatment,
anticipating how and why microbes will evolve in response to their environments (as manipulated by humans).
Ewald (1994) suggests that we might actually be able to manipulate evolution of disease microbes in ways
that would benefit humans. In their excellent book "Evolution and Healing" (unfortunately given the dumbed-down
title "Why we get sick" for American audiences), Nesse and Williams argue that natural selection has molded
a wide variety of human emotions such as anxiety, sadness and depression -- such traits could well be
adaptations that enhance fitness.
Microbiomes.
You are composed of about 100 trillion cells, but only 10 trillion are your own cells. Most of the other
90 trillion are bacteria, with a few other parasites, fungi, and nifty other little creatures, mixed in.
The eyebrow mite Demodex folliculorum lives in your bushy eyebrow forests and burrows into eyebrow hair
follicles but causes little harm. Your health depends on many of these commensals. Some can cause cancers,
others lead to allergies, asthma, diabetes, and obesity, and still others control appetite and enhance or
inhibit immune function. Some of our contingent of microbes affect neurotransmitters and hence brain function
as well as contribute to our moods and general sense of well being.
As many as ten thousand different species of bacteria exploit our bodies as favorable environmental substrates,
one of the most important being the coliform human gut bacterium Escherichia coli, named for where they
live: in our colons. Each of us houses 6 billion of these tiny commensal microbes, almost as many bacteria as
there are people on planet Earth. They are not parasites but without them in our intestines, other less benevolent
parasitic bacteria like Salmonella invade. When you lose your E. coli population, say by taking
antibiotics, you get diarrhea. E. coli are vital to our well being because they keep our guts functional.
Antibiotics can save lives but they also can also cause collateral damage by taking out beneficial gut bacteria.
Indeed our good bacteria may well act as living antibiotics that protect us from harmful microbes. Many modern
ailments, including allergies, diabetes, and obesity, are now thought to stem from incomplete
microbiomes
that have been reduced by antibiotics. When prescribing antibiotics, many doctors also recommend that patients take
probiotics to replenish their intestinal microbiome.
Coevolution
In its broadest sense,
coevolution
refers to the joint evolution of two (or more) taxa that have close ecological relationships but do not exchange genes, and in which reciprocal selective pressures operate to make the evolution of either taxon partially dependent on the evolution of the other (Ehrlich and Raven 1964). Thus, coevolution includes most of the various forms of population interaction, from competition to predation to mutualism. An example of tight coevolution between pinworm parasites and their primate hosts is shown in Figure 15.11.
-
Figure 15.11. Parallel phylogenies of the nematode genus Enterobius pinworms and their primate hosts. [From Mitter and Brooks (1983) after Brooks and Glen (1982).]
The term coevolution is also often used in a more restricted sense to refer primarily to the interdependent evolutionary interactions between plants and animals, especially their herbivores and pollinators. A plant may evolve a secondary chemical substance that deters the vast majority of predators, but if a particular herbivore can in turn evolve a physiological means of coping with the chemical deterrent, it can thereby obtain an uncontested food supply. Through this kind of coevolution, many herbivores have
become strongly specialized on a single species or a few closely related species of plants. Thus, Drosophila pachea is the only species of fruit fly that can exploit the "senita" group of cacti; these plants produce an alkaloid that is fatal to the larvae of all other fruit flies, but D. pachea has apparently evolved a means of detoxifying this chemical (Kircher et al. 1967).
In many cases, such specialized herbivores even use a plant's toxic chemicals (often aromatic and quite pungent) as cues in locating and/or selecting their host plants. Some herbivores, such as the monarch butterfly, actually sequester plant poisons (cardiac glycosides in this case), which in turn make the herbivore itself unpalatable or even poisonous to its own potential predators.
Danaid butterflies and certain moths make double use of polyuridine alkaloids -- these noxious chemicals are not only sequestered by larvae and/or adults and used for antipredator purposes but are also exploited as chemical precursors in the synthesis of pheromones important for mate attraction. An arginine mimic, l-canavanine, present in many legumes, ruins protein structure in most insects; however, a bruchid beetle has evolved metabolic machinery that enables it to utilize plants containing canavanine.
Attempts have been made to generalize about the coevolution of herbivores and plant antiherbivore tactics (Cates and Orians 1975). Feeny (1975) argues that rare or ephemeral plant species are hard for herbivores to find and hence are protected by escape in time and space; moreover, he asserts, such unapparent plant species should evolve a diversity of qualitatively different, chemically inexpensive defenses that should constitute effective evolutionary barriers to herbivory by nonadapted generalized herbivores that are most likely to find such "cryptic" plants. However, such qualitative defenses will be only minimal ecological barriers to adapted specialized herbivores, against which the plant's primary antiherbivore tactic is escape in time and space (i.e., not being found). In contrast, Feeny reasons that abundant and/or persistent plant species cannot prevent herbivores from finding them either in ecological or evolutionary time. Such "apparent" plant species appear to have evolved more expensive quantitative defenses (named that because they are more effective in higher doses). Examples include tough leaves of low nutrient or water content containing large amounts of relatively nonspecific chemicals such as tannins (Table 15.3). Feeny points out that such plant defenses should pose a significant ecological barrier to herbivores, although perhaps only a weak evolutionary barrier unless supplemented with qualitative chemical defenses (some plants have both).
Table 15.3 Some of the Suggested Correlates of Plant Apparency
______________________________________________________________________________
Apparent Plants ![]() Unapparent Plants Unapparent Plants
______________________________________________________________________________
Common or conspicuous![]() Rare or ephemeral Rare or ephemeral
Woody perennials ![]() Herbaceous annuals Herbaceous annuals
Long leaf life span![]() Short-lived leaves Short-lived leaves
Slow growing, competitive species![]() Faster growing, often fugitive species Faster growing, often fugitive species
Late stages of succession, climax![]() Early stages of succession, second growth Early stages of succession, second growth
Bound to be found by herbivores![]() Protected from herbivores by escape in Protected from herbivores by escape in
(cannot escape in time and space)![]() time and space (but still encountered by time and space (but still encountered by
![]() wide-ranging generalized herbivores) wide-ranging generalized herbivores)
Produce more expensive quantitative![]() Produce inexpensive qualitative chemical Produce inexpensive qualitative chemical
(broad-based) antiherbivore defenses![]() defenses (poisons or toxins) to discourage defenses (poisons or toxins) to discourage
(tough leaves, thorns, tannins)![]() generalized herbivores generalized herbivores
Quantitative defenses constitute![]() Qualitative defenses may be broken down Qualitative defenses may be broken down
effective ecological barriers to her-![]() over evolutionary time by coevolution of over evolutionary time by coevolution of
bivores, although perhaps only a weak![]() appropriate detoxification mechanisms in appropriate detoxification mechanisms in
evolutionary barrier unless supple-![]() herbivores (host plant-specific herbivore herbivores (host plant-specific herbivore
mented with qualitative defenses![]() species result) species result)
______________________________________________________________________________
Cates and Orians (1975) develop somewhat different but related predictions for early versus late successional plant species. Because early successional plants escape from herbivores in space and time, Cates and Orians reason that such plants should allocate fewer resources to chemical antiherbivore defenses than the more apparent plants of later stages in succession. Thus, early successional plant species should make better foods for generalized herbivores than later successional and climax plant species. Indeed, experimental studies on slug feeding indicate that early successional annuals were significantly more palatable than later successional species (Cates and Orians 1975). However, the opposite result was obtained by Otte (1975) in similar experiments with grasshoppers; these generalized herbivores accepted more later successional plant species than early ones. Otte suggests that this difference may arise from the difference in mobility between slugs and grasshoppers. In a survey of lepidopteran feeding habits, Futuyma (1976) found greater degrees of specialization to host plant species among insects feeding on herbaceous plants than among those that feed on leaves of shrubs and trees (this pattern neatly fits Feeny's plant apparency dichotomy). Futuyma suggests that plant defense systems are more diverse in floristically rich plant communities than they are in less diverse communities. Discrepancies among these studies indicate that generalizations concerning plant apparency are difficult to make and that they will have to allow for exceptions (for further discussion of this interesting area, see Rhoades and Cates 1976; Gilbert 1979; Futuyma and Slatkin 1983; and Coley et al. 1985).
Populations of wild ginger, Asarum caudatum, in western Washington are polymorphic for growth rate, seed production, and palatability to a native slug, Ariolimax columbianus (Cates 1975). In habitats where slugs were low in abundance, Cates found that populations of wild ginger were dominated by individuals allocating more energy to growth and seed production and less to production of antiherbivore chemicals. Presumably, less palatable plants have a fitness advantage in habitats with more slugs; even though they grow more slowly, they lose less photosynthetic tissue to slug herbivory.
Plants and fungi have evolved a wide variety of chemicals to fight microbes such as bacteria that attack them. Antibiotics were first discovered in fungi, but have now also been found in many species of plants as well. Secondary chemicals of plants have proven to be a vast reservoir for useful pharmaceutical products. These include analgesics, diuretics, laxatives, tranquilizers, contraceptive pills, and cough drops.
Clinically proven drugs derived from higher plants include morphine, codeine, atropine, quinine, digitalis, and many others. Bark of Pacific yew trees contains taxol, which has proven to be an effective agent in the treatment of certain ovarian cancers. To date, scientists have examined only about 1 percent of existing plant species for useful pharmacuticals.
In some cases, plants have actually formed cooperative relationships with animals that result in their protection from certain herbivore species. By means of ant removal experiments, Janzen (1966) showed that some species of Acacia deprived of their normal epiphytic ant fauna are highly palatable to herbivorous insects, whereas species that do not normally have ants for protection from herbivores are less palatable. These acacias benefiting from ant protection actually produce nectaries and swollen thorns that attract and in turn benefit the ants! Thus, these plants put matter and energy into attracting ants that defend their leaves, rather than into more direct chemical warfare. This antiherbivore ploy is broad based, since the ants ferociously attack a wide range of herbivores. Ant protection may also reduce competition with other plants, as well as provide a measure of protection from fire, because the ants keep the ground surface clear immediately around their Acacia plant.
Many plants protect their seeds either by enclosing them in a toxic matrix and/or by means of a hard shell. Some seeds are poisonous. Nevertheless the high nutrient content of seeds has resulted in the evolution of effective seed predators. Predation on seeds may often be heaviest where they occur in greatest concentrations (such as acorns underneath a parent oak) because seed predator populations will generally be largest where the most food is available (Janzen 1971b). As a result, the probability of an individual seed's surviving to establish itself as a plant may often vary inversely with seed density. In many trees, most seeds fall to the ground near the parent tree, with a continually decreasing number of seeds ending up at distances farther from the parent tree. As a result of these opposing processes, Janzen suggests that recruitment is maximized at some distance from the parent tree (Figure 15.12). Janzen's seedling ring model of seed predation and recruitment may help to explain the high species diversity of tropical trees, which suffer heavy seed losses to specialized seed predators that eat the seeds of particular tree species. (It neglects the question of why there are so many specialized seed predators in the tropics.)
-
Figure 15.12. Hypothetical model of seed recruitment versus distance from the parent tree. Near the tree, all seeds are eaten by seed predators; at increasing distances from the tree, the probability of seed survival increases as the density of seeds and their predators decreases. Although the number of seeds drops with distance from the parental tree, recruitment is highest at some distance from the tree. A ring of seedlings should result. [After Janzen (1970).]
Intricacies of coevolutionary relationships between pine squirrels (Tamiasciurus) and their coniferous food trees were studied in the Pacific Northwest by C. Smith (1970). Conifer seeds constitute the staple food supply of these squirrels; they can effectively strip a tree of most of its cones. Trees reduce the effectiveness of squirrel predation in many different ways: (1) by producing cones that are difficult for the squirrels to reach, open, and/or carry; (2) by putting fewer seeds into each cone (squirrels eat only the seeds themselves and must "husk" cones to get them); (3) by increasing the thickness of seed coats, requiring that the squirrels spend more time and energy extracting each seed; (4) similarly, by putting less energy into each seed (a drawback is that seedlings from smaller seeds have fewer resources at their disposal and are presumably poorer competitors than seedlings from larger seeds); (5) by shedding seeds from cones early, before the young squirrels of the year begin foraging; and (6) by periodic cone "failures" that decimate the squirrel population, thereby reducing the intensity of predation during the next year. Thus, squirrel predation has had profound evolutionary influences upon various reproductive characteristics of conifers, including details of cone anatomy and location, the number of seeds per cone (and the variability in the number per cone), the time at which the cones shed their seeds, the thickness of seed coats, and annual fluctuations in the size of cone crops. Evolution of these defense mechanisms by the conifers has in turn forced squirrels to adapt in various ways, such as choosing cones carefully and stockpiling them.
Fluctuations in cone crops from year to year are pronounced and are best interpreted as an antisquirrel strategy, because they occur even when climatic conditions are apparently favorable for trees. Apparently, the conifers withhold their products of primary production and store them for later use. Cone crops and failures are often synchronized among different tree species in a given area -- a further indication that the phenomenon is directed at the squirrels. Individual trees out of synchrony presumably set fewer seeds and are thus selected against by natural selection. Smith points out that different conifer species have diverged from one another in evolution of cone anatomy, size, location, and time of shedding but that these same conifer species have converged in their fluctuations in cone crops. Both reduce the squirrel's efficiency.
Selected References
Predation
Errington (1946); Holt (1977); Janzen (1971b); MacArthur (1972); MacArthur and Connell (1966); Schall and Pianka (1980); Wilson and Bossert (1971).
Predator-Prey Oscillations
Elton (1942); Errington (1946); Gause (1934, 1935); Haigh and Maynard Smith (1972); Hassell (1980); Holling (1959a, b, 1961, 1966); Keith (1963); Leslie (1948); Leslie and Gower (1960); Levins (1966); Lotka (1925); Maynard Smith (1974); Pielou (1969, 1974); Rosenzweig (1971, 1973a, b); Rosenzweig and MacArthur (1963); Solomon (1949); Tanner (1975); Volterra (1926a, b, 1931); Wangersky and Cunningham (1956); Wilson and Bossert (1971).
"Prudent" Predation and Optimal Yield
Beverton and Holt (1957); Gilpin (1975a); MacArthur (1960b, 1961); Slobodkin (1968).
Selected Experiments and Observations
Errington (1946, 1956, 1963); Force (1972); Gause (1934, 1935); Holling (1959a, 1965); Holyoak and Lawler (1996a, 1996b); Huffaker (1958); Luckinbill (1973, 1974); Maly (1969); Menge (1972a); Murdoch (1969); Neill (1972); Paine (1966); Salt (1967); Utida (1957); Wilbur (1972).
Evolutionary Consequences: Prey Escape Tactics
Benson (1933); Benson (1972); Benson, Brown, and Gilbert (1975); Cott (1940); Ehrlich and Raven (1964); Feeny (1968, 1970); Fisher (1958b); Gordon (1961); Holt (1977); Janzen (1966, 1967, 1970, 1971b); Jeffries and Lawton (1985); Jones (1962, 1966); Kettlewell (1956, 1958); McKey (1974); Otte and Williams (1972); Rand (1967); Ricklefs and O'Rourke (1975); Schall and Pianka (1980); Sheppard (1959); Stahl (1888); Stradling (1976); Whittaker and Feeny (1971).
Parasitism
Anderson (1981); Anderson and May (1979, 1982); Barbahenn (1969); Bradley (1972, 1974); Cornell (1974); Crofton (1971a, b); Damian (1964, 1979); Esch (1977); Esch, Hazen, and Aho (1977); Ewald (1983, 1987, 1994); Fallis (1971); Gillespie (1975); Haldane (1941); Hirsch (1977); Holmes (1973, 1983); Holmes and Bethel (1972); Jennings and Calow (1975); Kennedy (1975); May and Anderson (1979); Moore (1987); Park (1948); Price (1980, 1988); Rohde (1979); Schad (1963, 1966); Schall (1982, 1992); Toft (1986); Toft and Karter (1990); Toft et al. (1991); Turner (1980); Warner (1968).
Epidemiology
Anderson (1976, 1981); Anderson and May (1979, 1982); Bailey (1975); Bartlett (1960); Gillespie (1975); Haldane (1949); May and Anderson (1979).
Darwinian Medicine
Brandts et al. (1997); Ewald (1995); Neese and Williams (1994); Williams and Neese (1991).
Coevolution
Bernays and Chapman (1994); Brooks and Glen (1982); Brower (1969); Brower and Brower (1964); Brues (1920); Caswell et al. (1973); Cates (1975); Cates and Orians (1975); Caughley and Lawton (1981); Chambers (1970); Coley et al. (1985); Dressler (1968); Ehrlich and Raven (1964); Faegri and van der Pijl (1971); Feeny (1970, 1975, 1976); Feinsinger (1983); Fraenkel (1959); Freeland and Janzen (1974); Futuyma (1976); Futuyma and Slatkin (1983); Gilbert (1971, 1972, 1979); Gilbert and Raven (1975); Gilbert and Singer (1973, 1975); Gordon (1961); Heinrich and Raven (1972); Howe (1984); Janzen (1966, 1967, 1971a, 1971b); Kiester et al. (1985); Kircher and Heed (1970); Kircher et al. (1967); Lawlor and Maynard Smith (1976); Mitter and Brooks (1983); Otte (1975); Price (1975); Rathcke (1979); Rhoades (1979, 1985); Rhoades and Cates (1976); Rick and Bowman (1961); C. Smith (1970); Thompson (1982, 1989); Willson (1973c).
|

