14| Experimental Ecology
Design of Experiments
Experiments are designed to test hypotheses, typically based on simple causality. Ecological experiments are usually difficult to perform, even without insistence on proper statistical procedures. Because random and directed changes will probably be taking place during the time course of an experiment, a so-called control, a nearby untreated area, is essential for comparison with the manipulation. Such control plots should be randomly intermixed with treatment plots, both in space and in time. Moreover, both the control and the experiment must be replicated enough times to convince a statistician that there is a significant difference between the controls and the experiments. If such replicates are not truly independent of one another, the experimenter is committing pseudoreplication (Hurlbert 1984). Few experiments in ecology can meet these standards and many are not replicated at all. Moreover, multiple causality and indirect effects make the unexpected results of many experiments difficult to interpret.
Ecological Experiments
Although difficult, various manipulations of natural populations involving species removals, additions, and/or transplants have been informative. Terrestrial plants and relatively immobile animals, such as marine invertebrates in the rocky intertidal, are best suited for such studies.
A potent technique for studying the effects of predation in nature is to exclude predators from an area and then compare this experimental site with an adjacent "control" area, which is similar but unaltered, with normal access for predators. Hopefully, resulting differences between the two areas can best be ascribed to predation. Paine (1966) performed such a predator removal experiment along the rocky intertidal seacoast of the Olympic Peninsula in Washington State. When the major top predator, the sea star Pisaster ochraceus, was removed, the number of species remaining was drastically reduced. Control areas with Pisaster supported some 15 species of marine invertebrates, but the area without the starfish had only 8 species. The rocky intertidal is a space-limited system. In the absence of predation, more efficient occupiers of space (so-called competitive dominants), especially the bivalve Mytilus californianus (a mussel), dominated the area. Presumably, by preying on Mytilus, Pisaster continually open up new patches that are rapidly colonized by fugitive species that are less efficient competitors for space. Thus, by reducing the level of competition at lower trophic levels, this starfish predator allows coexistence of otherwise competitively incompatible species. Such keystone predators may have a powerful impact on community structure. The importance of the keystone species concept has been disputed (Mills et al. 1993; Power et al. 1996; Hurlbert 1997).
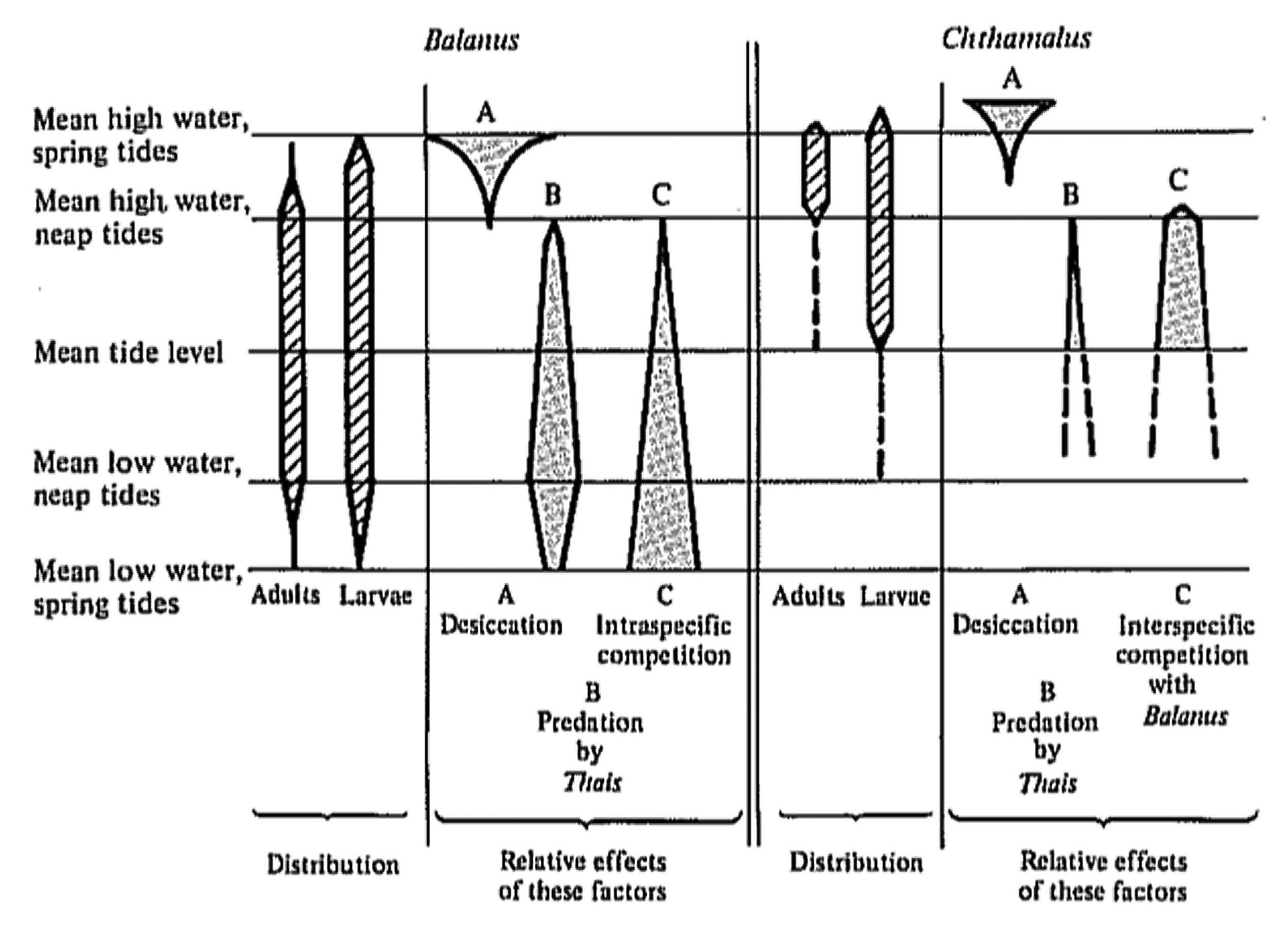
Competition for space between two species of barnacles, Balanus balanoides and Chthamalus stellatus, was studied by Connell (1961a, b) along a rocky Scottish seacoast. These two crustaceans occupy sharply defined horizontal zones, as do most sessile organisms in the marine intertidal, with Chthamalus occupying an upper zone, and Balanus, a lower zone (Figure 14.1). Larvae of both species settle and attach over a wider vertical range than the zone occupied by adults. By manipulation of wire cages to exclude a snail predator (Thais) and by periodic removal of barnacles, Connell elucidated the various forces causing the zonation. He demonstrated that although Chthamalus loses in competition with Balanus, adults persist in a narrow band because Chthamalus are more tolerant of physical desiccation than Balanus. Connell suggests that the lower limit of distribution of intertidal organisms is usually determined mainly by biotic factors, such as competition with other species and predation, whereas the upper limit is more often set by physical factors, such as dry conditions prevailing during low tides.
-
Figure 14.1. Vertical distributions of larvae and adults of two barnacle species, Balanus balanoides and Chthamalus stellatus, in the rocky intertidal of Scotland. Relative intensities of various limiting factors are represented diagrammatically by widths of shaded areas. [After Connell (1961b). By permission of Duke University Press.]
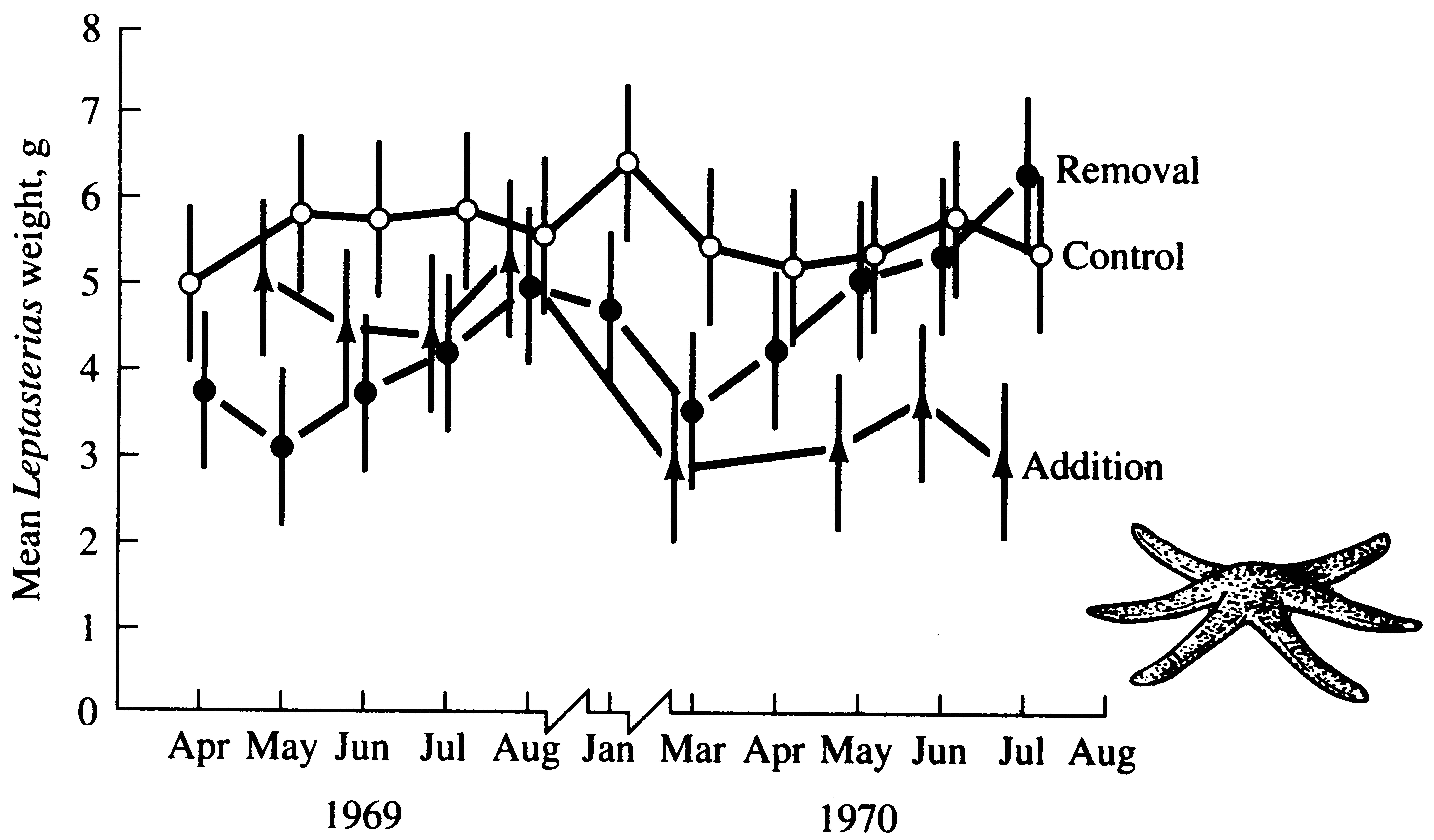
In another such field experiment, all individuals of the large intertidal sea star Pisaster ochraceus were removed from a small island reef and added to another similar reef, while a third undisturbed nearby reef was monitored as a "control" (Menge 1972b). A smaller starfish, Leptasterias hexactis, whose diet overlaps broadly with that of Pisaster (thus a potential competitor for food), was present on all three reefs. The average biomass of individual Leptasterias increased significantly with removal of Pisaster and decreased with its addition, while the size of control Leptasterias remained constant (Figure 14.2). Estimated standing crops of the two species of sea stars varied inversely over a series of study areas (Figure 14.3), further implicating interspecific competition.
-
Figure 14.2. Response of individual Leptasterias (wet weights) to the removal or addition of Pisaster sea stars, with control. [From Menge (1972).]
-
Figure 14.3. Inverse correlation between the biomasses of Pisaster and Leptasterias across ten sites in Puget Sound. [After Menge (1972).]
In another field manipulation, Dunham (1980) studied competition between two species of rock-dwelling iguanian lizards at Big Bend National Park in Texas using similar, but reciprocal removal experiments. In two dry years, food supplies were apparently scant and removal of the larger lizard species (Sceloporus merriami) had numerous significant effects on the smaller species (Urosaurus ornatus), including increases in density, feeding success, growth rates, lipid levels, and prehibernation body weights. Treatments did not differ from controls during the two wet years, when insect food resources were presumably superabundant. Only one effect of the smaller species on the larger one was evident in these removal experiments -- Sceloporus survival was significantly higher in one of the two dry years. Clearly, competition is not reciprocal and varies in intensity from year to year.
One of the more ambitious experiments ever undertaken in ecology was reported by Munger and Brown (1981) and Brown et al. (1986). These investigators undertook labor-intensive manipulations to tease apart the interactions occurring among seed plants and their seed-eating predators, heteromyid rodents and ants, in the Chihuahuan desert in extreme southeastern Arizona. At a reasonably homogeneous flatland desert site, twenty-four 50 x 50m (0.25 hectare) plots were fenced off. Small holes (1.9cm diameter) at ground level in these fences allowed ingress and egress for small rodents but physically excluded all kangaroo rats (Dipodomys). (These fences were likened to "semi-permeable membranes.") Control plots had large 6.5cm diameter holes that allowed free movement of all rodents including Dipodomys. Rodents were removed by live trapping and ants by poisoning. In addition, 4 seed addition treatments allowed inferences about the effects of seeds on seed predators. Eight plots had holes in their fences, 8 were poisoned, and 8 were seed addition treatments (Table 14.1). Eleven different experimental manipulations were each replicated once at random (i.e., in two plots). The remaining two plots were untreated "controls" (Table 14.1).
Rodent removals resulted in an increase in Pheidole xerophila, a small ant species. Seed addition resulted in increases in rodent densities. Removal of large rodents resulted in an increase in the densities of smaller rodents. On plots where all species of rodents were removed, large-seeded species of plants gained at the expense of small-seeded species. In the short-term, rodents and ants competed for seeds. However, over the long term (treatments were monitored for 10 years!), ants indirectly benefited rodents via facilitation: rodents prefer large seeds and ants prefer small seeds, but because large-seeded plants compete with small-seeded plants, the presence of rodents indirectly benefits ants. The direct impact of ants on rodents, however, appears to be negligible.
Table 14.1 Experimental Design of Seed Predation in the Chihuahuan Desert
__________________________________________________________________________
Plots![]() Treatments
Treatments
__________________________________________________________________________
11,14 Controls
Controls
6,13 Seed addition, large seeds, constant rate
Seed addition, large seeds, constant rate
2,22 Seed addition, small seeds, constant rate
Seed addition, small seeds, constant rate
9,20 Seed addition, mixed seeds, constant rate
Seed addition, mixed seeds, constant rate
1,18 Seed addition, mixed seeds, temporal pulse
Seed addition, mixed seeds, temporal pulse
5,24 Rodent removal, Dipodomys spectabilis (largest kangaroo rat)
Rodent removal, Dipodomys spectabilis (largest kangaroo rat)
15,21 Rodent removal, all Dipodomys species (kangaroo rats)
Rodent removal, all Dipodomys species (kangaroo rats)
7,16 Rodent removal, all seed-eating rodents
Rodent removal, all seed-eating rodents
8,12 Pogonomyrmex harvesting ants
Pogonomyrmex harvesting ants
4,17 All seed-eating ants
All seed-eating ants
3,19 All Dipodomys plus Pogonomyrmex ants
All Dipodomys plus Pogonomyrmex ants
10,23 All seed-eating rodents plus all seed-eating ants
All seed-eating rodents plus all seed-eating ants
__________________________________________________________________________
Source: From Brown et al. (1986).
Hundreds of such manipulative field experiments have now been undertaken to demonstrate the importance of both competition and predation, although their design is too often somewhat less than perfect (Hairston 1989; Underwood 1986). Schoener (1983) reviewed the extensive literature on competition experiments, which include both aquatic and terrestrial as well as plant and animal studies; a full 90 percent of the 164 studies and 76 percent of the species examined exhibit evidence of interspecific competition. In a similar review of predation removal-addition experiments, Sih et al. (1985) reported even higher percentages of studies demonstrating significant impact of predators.
Such experiments suffer from at least two major shortcomings. First, failure to detect a niche shift in response to removal of a potential competitor may simply be an indication that the target population is not ecologically flexible (the idea that species have become evolutionarily locked into particular adaptive zones due to competition in the historical past has been termed "the ghost of competition past" by Rosenzweig (1979) and others). Likewise, predator removal experiments can fail in systems with donor control where predators consume only prey that would die anyway from other causes. Second, and perhaps of greater interest and importance, the extent to which indirect effects mediated through other members of a community network act in opposition to the direct pairwise interaction is not easily determined (Bender et al. 1984). Indeed, Sih et al. (1985) reported surprisingly high numbers of "unexpected" results, which could well stem from indirect interactions (Chapter 11).
A Defaunation Experiment
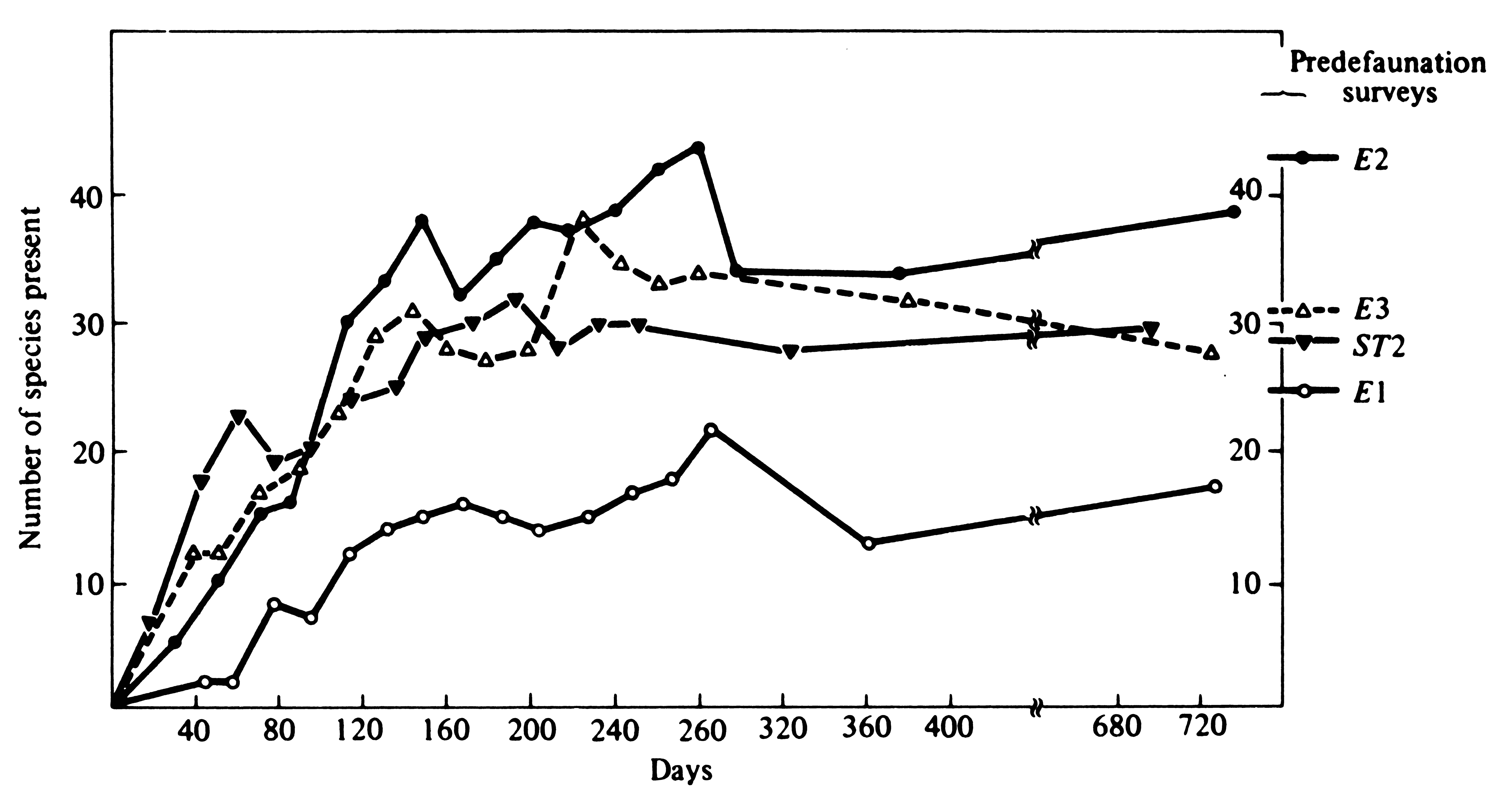
An interesting ecological experiment was performed by Simberloff and Wilson (1970 and included references). After censusing the entire arthropod faunas of several very tiny mangrove islets in the Florida Keys, all arthropods were exterminated by fumigation with a deadly gas (methyl bromide). The vegetation survived relatively unscathed, but even insect eggs were killed. The process of recolonization was then monitored over a two-year period. Arthropods rapidly recolonized; within a mere 200 days, numbers of species on the islets had stabilized (Figure 14.4). Although turnover rates remained quite high throughout the experimental period, numbers of species on the islets remained relatively constant over a period of nearly two years, providing strong evidence that the islands have indeed reached an equilibrium. Two islands, E1 and E2, seem to have reached equilibrium at a slightly lower species density after defaunation.
-
Figure 14.4. Colonization curves (number of arthropod species through time) of four tiny mangrove islets in the Florida keys following complete extermination of the arthropod fauna, which resulted in relatively little damage to the vegetation. Numbers of species on each islet first rose rapidly, then soon leveled off to species densities fairly close to those before defaunation (plotted on the right vertical axis). [After Simberloff and Wilson (1970). By permission of Duke University Press.]
The apparent depression in the number of species at equilibrium may indicate that the species that reinvaded these islands interfere with one another more than the members of the original communities did; alternatively, this reduction in species density may be due to the fact that new immigrants are not adapted to exploit the island's resources as well as were the original inhabitants. In any case, these results demonstrate that the actual composition of an island's fauna may itself partially determine the equilibrium number of species an island will support.
An instructive analysis of these data was undertaken by Heatwole and Levins (1972), who tallied up the numbers of species of insects in various (somewhat arbitrary) trophic categories before and after defaunation (Table 14.2). The apparent return to a similar state could be evidence of resiliency and suggests that an equilibrium trophic structure might exist. However, the species pool of potential invaders contains many more species of herbivorous insects, ants, and other carnivores than scavengers, detritivores, wood borers, or parasites, so that this apparent "return to an equilibrium state" could also arise as an artifact of sampling (Simberloff 1976; May 1981).
Table 14.2 Evidence for Stability of Trophic Structure?
_________________________________________________________________________________________
 Trophic Classes
Trophic Classes
________________________________________________________________________________________
Island H
H S
S D
D W
W A
A C
C P
P ?
? Total
Total
_________________________________________________________________________________________
E1 9(7)
9(7) 1(0)
1(0) 3(2)
3(2) 0(0)
0(0) 3(0)
3(0) 2(1)
2(1) 2(1)
2(1) 0(0)
0(0) 20(11)
20(11)
E2 11(15)
11(15) 2(2)
2(2) 2(1)
2(1) 2(2)
2(2) 7(4)
7(4) 9(4)
9(4) 3(0)
3(0) 0(1)
0(1) 36(29)
36(29)
E3 7(10)
7(10) 1(2)
1(2) 3(2)
3(2) 2(0)
2(0) 5(6)
5(6) 3(4)
3(4) 2(2)
2(2) 0(0)
0(0) 23(26)
23(26)
ST2 7(6)
7(6) 1(1)
1(1) 2(1)
2(1) 1(0)
1(0) 6(5)
6(5) 5(4)
5(4) 2(1)
2(1) 1(0)
1(0) 25(18)
25(18)
E7 9(10)
9(10) 1(0)
1(0) 2(1)
2(1) 1(2)
1(2) 5(3)
5(3) 4(8)
4(8) 1(2)
1(2) 0(1)
0(1) 23(27)
23(27)
E9 12(7)
12(7) 1(0)
1(0) 1(1)
1(1) 2(2)
2(2) 6(5)
6(5) 13(10)
13(10) 2(3)
2(3) 0(1)
0(1) 37(29)
37(29)
Totals 55(55)
55(55) 7(5)
7(5) 13(8)
13(8) 8(6)
8(6) 32(23)
32(23) 36(31)
36(31) 12(9)
12(9) 1(3)
1(3) 164(140)
164(140)
_________________________________________________________________________________________
Note: Table is after Heatwole and Levins (1972). Islands are labeled as in Simberloff and Wilson's (1969) original notation, and on each the fauna is classified into trophic groups: herbivore (H), scavenger (S), detritus feeder (D), wood borer (W), ant (A), carnivorous predator (C), parasite (P), class undetermined (?). First figures are number of species before defaunation, figures in parentheses are corresponding numbers after recolonization. Total number of different species encountered in the study was 231 (the simple sum 164 + 140 counts some species more than once).
Wilson (1969) suggests that an island community may experience a sequence of several distinct sorts of equilibria through time (Figure 14.5). First, a "noninteractive" equilibrium in the number of species present may be reached even before component populations come to equilibrium demographically and with one another. Then, because competitive and predatory interactions are intensified as populations saturate the island with individuals, a second "interactive" equilibrium is reached. Both stages should occur relatively rapidly. Wilson (1969) envisions two other types of equilibria, which would
-
Figure 14.5. Hypothetical sequence of community equilibria on an island through time. The time scale is arbitrary and intended only to emphasize the vastly greater time spans required for the assortative and evolutionary equilibria (see text). [From Wilson (1969).]
require considerably greater time spans to attain. As various species go extinct and others invade, the composition of an island's biota may gradually change until a certain set of species from the available species pool is reached that is composed of species with particularly low extinction rates; Wilson (1969) terms this the "assortative" equilibrium. Finally, given a much longer time span, the component species could actually evolve minimal extinction rates and an "evolutionary" equilibrium might be reached.