5| Resource Acquisition and Allocation
Limiting Factors and Tolerance Curves
Ecological events and their outcomes, such as growth, reproduction, photosynthesis, primary production,
and population size, are often regulated by the availability of one or a few factors or requisites in short
supply, whereas other resources and raw materials present in excess may go partially unused. This principle
has become known as the "law of the minimum" (Liebig 1840).
For instance, in arid climates, primary production (the amount of solar energy trapped by green plants) is
strongly correlated with precipitation (Figure 5.1); here water is a "master limiting factor." Of many different factors that can be limiting, frequently among the most important are various nutrients, water, and temperature.
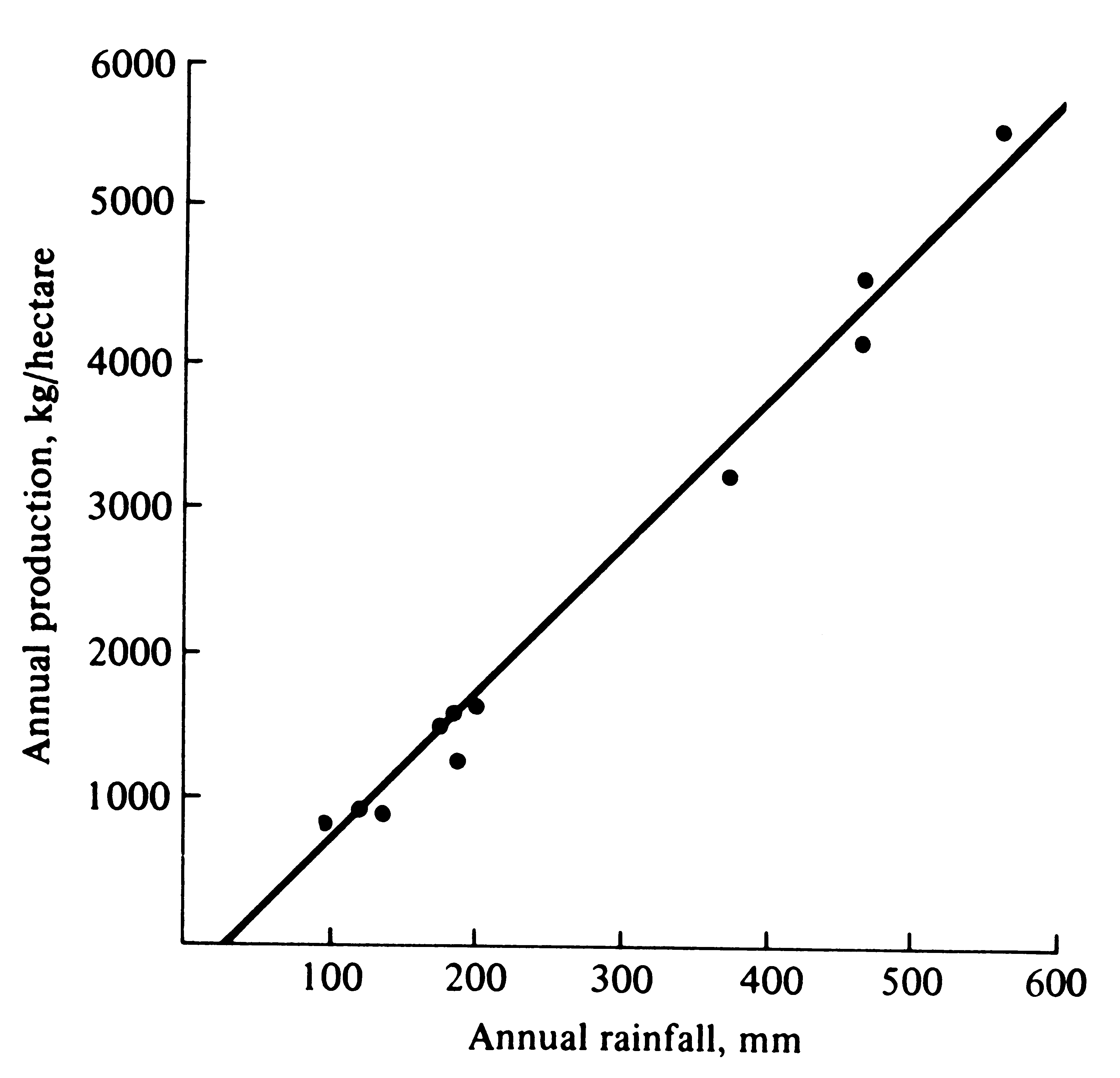 Figure 5.1. An example of the strong correlation between annual rainfall and primary production along a precipitation gradient in a desert region of Namibia. [Adapted from Odum (1959) after Walter (1939).]
Figure 5.1. An example of the strong correlation between annual rainfall and primary production along a precipitation gradient in a desert region of Namibia. [Adapted from Odum (1959) after Walter (1939).]
When considering populations, we often speak of those that are food-limited, predator-limited, or climate-limited. Populations may be limited by other factors as well; for example, density of breeding pairs of blue tits (Parus caeruleus) in an English woods was doubled by the addition of many new nesting boxes (Lack 1954, 1966), providing an indication that nest sites were limiting. However, limiting factors are not always so clear-cut but may usually interact so that a process is limited simultaneously by several factors, with a change in any one of them resulting in a new equilibrium. For instance, both increased food availability and decreased predation pressures might result in a larger population size.
A related concept, developed by Shelford (1913b), is now known as the "law of tolerance." Too much or too little of anything can be detrimental to an organism. In the early morning, a desert lizard finds itself in an environment that is largely too cold, whereas later in the day its environment is too hot. The lizard compensates somewhat for this by spending most of its time during the early morning in sunny places, whereas later on most of its activities take place in the shade. Each lizard has a definite optimal range of temperature, with both upper and lower limits of tolerance. More precisely, when measures of performance (such as fitness, survivorship, or foraging efficiency) are plotted against important environmental variables, bell-shaped curves usually result (for examples, see Figures 5.2 and 5.8).
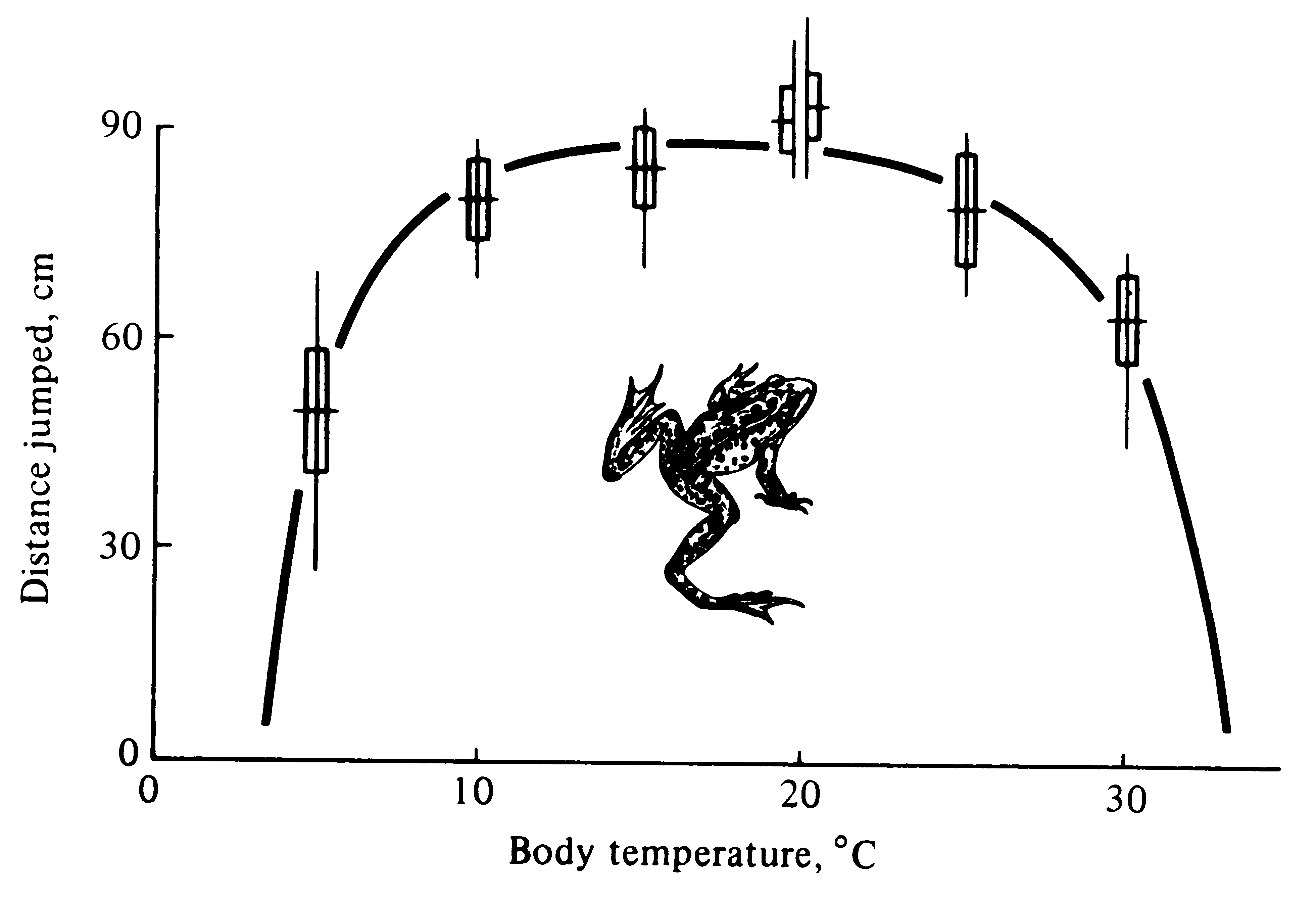 Figure 5.2. Distance jumped by a frog as a function of its body temperature. Notice that performance diminishes at both low and high temperatures. [From Huey and Stevenson (1979).]
Figure 5.2. Distance jumped by a frog as a function of its body temperature. Notice that performance diminishes at both low and high temperatures. [From Huey and Stevenson (1979).]
Organisms can be viewed as simple input-output systems, with foraging or photosynthesis providing an input of materials and energy that are in turn "mapped" into an output consisting of progeny. Fairly extensive bodies of theory now exist both on optimal foraging and reproductive tactics (see next chapter). In optimal foraging theory, the "goal" usually assumed to be maximized is energy uptake per unit time (successful offspring produced during an organism's lifetime would be a more realistic measure of its foraging ability, but fitness is exceedingly difficult to measure). Similarly, among organisms without parental care, reproductive effort has sometimes been estimated by the ratio of calories devoted to eggs or offspring over total female calories at any instant (rates of uptake versus expenditure of calories have unfortunately not yet infiltrated empirical studies of reproductive tactics). To date, empirical studies of resource partitioning have been concerned largely with "input" phenomena such as overlap in and efficiency of resource utilization and have neglected to relate these to "output" aspects. In contrast, empirical studies of reproductive tactics have done the reverse and almost entirely omitted any consideration of foraging. Interactions and constraints between foraging and reproduction have barely begun to be considered. A promising area for future work will be to merge aspects of optimal foraging with optimal reproductive tactics to specify rules by which input is translated into output; optimal reproductive tactics ("output" phenomena) surely must often impose substantial constraints upon "input" possibilities and vice versa.
Resource Budgets and the Principle of Allocation
Any organism has a limited amount of resources available to devote to foraging, growth, maintenance, and reproduction. The way in which an organism allocates its time and energy and other resources among various conflicting demands is of fundamental interest because such apportionments provide insight into how the organism copes with and conforms to its environment. Moreover, because any individual has finite resource and energy budgets, its capacity for regulation is necessarily limited. Organisms stressed along any one environmental variable are thus able to tolerate a lesser range of conditions along other environmental variables. Various tolerance and performance curves (Figure 5.2) are presumably subject to certain constraints. For example, their breadth (variance) usually cannot be increased without a simultaneous reduction in their height and vice versa (Levins 1968). This useful notion of trade-offs, known as the principle of allocation, has proven to be quite helpful in interpreting and understanding numerous ecological phenomena.
As an example of allocation, imagine an animal of a given size and mouth-part anatomy. A certain size of prey item is optimal, whereas other prey are suboptimal because they are either too large or too small for efficient capture and swallowing. Any given animal has its own "utilization curve" that indicates the actual numbers of prey of different sizes taken per unit time under particular environmental conditions. In an idealized, perfectly stable, and infinitely productive environment, a utilization curve might become a spike with no variance, with the organism using only its most optimal prey resource type. In actuality, limited and changing availabilities of resources, in both time and space, result in utilization curves with breadth as well as height. In terms of the principle of allocation, an individual with a generalized diet adapted to eat prey of a wide range of available sizes presumably is not so effective at exploiting prey of intermediate size as another, more specialized, feeder. In other words, a jack-of-many-trades is a master of none. We will consider this subject in more detail later.
Time, Matter, and Energy Budgets
Time, matter, and energy budgets vary widely among organisms. For example, some creatures allot more time and energy to reproduction at any instant than do others. Varying time and energy budgeting is a potent means of coping with a changing environment while retaining some degree of adaptation to it. Thus, many male songbirds expend a great deal of energy on territorial defense during the breeding season but little or none at other times of the year. Similarly, in animals with parental care, an increasing amount of energy is spent on growing offspring until some point when progeny begin to become independent of their parents, whereupon the amount of time and energy devoted to them decreases. Indeed, adult female red squirrels, Tamiascurus, at the height of lactation consume an average of 323 kilocalories of food per day compared with an average daily energy consumption of a similar-sized adult male of only about 117 kilocalories (C. Smith 1968). The time budgets of these squirrels also vary markedly with the seasons.
In a bad dry year, many annual plants "go to seed" while still very small, whereas in a good wet year, these plants grow to a much larger size before becoming reproductive; presumably more seeds are produced in good years, but perhaps none (or very few) would be produced in a bad year if individuals attempted to grow to the sizes they reach in good years.
An animal's time and energy budget provides a convenient starting point for clarifying some ways in which foraging influences reproduction and vice versa. Any animal has only a certain finite period of time available in which to perform all its activities, including foraging and reproduction. This total time budget, which can be considered either on a daily basis or over the animal's lifetime, will be determined both by the diurnal rhythm of activity and by the animal's ability to "make time" by performing more than one activity at the same time (such as a male lizard sitting on a perch, simultaneously watching for potential prey and predators while monitoring mates and competing males). Provided that a time period is profitable for foraging (expected gains in matter and energy exceed inevitable losses from energetic costs of foraging), any increase in time devoted to foraging clearly will increase an animal's supply of matter and energy. However, necessarily accompanying this increase in matter and energy is a concomitant decrease in time available for nonforaging activities such as mating and reproduction. Thus, profits of time spent foraging are measured in matter and energy while costs take on units of time lost. Conversely, increased time spent on nonforaging activities confers profits in time while costs take the form of decreased energy availability. Hence, gains in energy correspond to losses in time, while dividends in time require reductions in energy availability. (Of course, risks of foraging and reproduction also need to be considered.)
The preceding arguments suggest that optimal allocation of time and energy ultimately depends on how costs in each currency vary with profits in the opposite. However, because units of costs and profits in time and energy differ, one would like to be able to convert them into a common currency. Costs and profits in time might be measured empirically in energetic units by estimating the net gain in energy per unit of foraging time. If all potential foraging time is equivalent, profits would vary linearly with costs; under such circumstances, the loss in energy associated with nonforaging activities would be directly proportional to the amount of time devoted to such activity. Optimal budgeting of time and energy into foraging versus nonforaging activities is usually profoundly influenced by various circadian and seasonal rhythms of physical conditions, as well as those of predators and potential prey. Clearly, certain time periods favorable for foraging return greater gains in energy gathered per unit time than other periods. Risks of exposure to both harsh physical conditions and predators must often figure into the optimal amount of time to devote to various activities. Ideally, one would ultimately like to measure both an animal's foraging efficiency and its success at budgeting time and energy by its lifetime reproductive success, which would reflect all such environmental "risks."
Foraging and reproductive activities interact in another important way. Many organisms gather and store materials and energy during time periods that are unfavorable for successful reproduction but then expend these same resources on reproduction at a later, more suitable, time. Lipid storage and utilization systems obviously facilitate such temporal integration of uptake and expenditure of matter and energy. This temporal component greatly complicates the empirical measurement of reproductive effort.
Prey density can strongly affect an animal's time and energy budget. Gibb (1956) watched rock pipits, Anthus spinoletta, feeding in the intertidal along the English seacoast during two consecutive winters. The first winter was relatively mild; the birds spent an average of 6-1/2 hours feeding, 1-3/4 hours resting, and 3/4 hour fighting in defense of their territory (total daylight slightly exceeded 9 hours). The next winter was much harsher and food was considerably scarcer; the birds spent 8-1/4 hours feeding, 39 minutes resting, and only 7 minutes on territorial defense! Apparently the combination of low food density and extreme cold (endotherms require more energy in colder weather) demanded that over 90 percent of the bird's waking hours be spent feeding and no time remained for frivolities. This example also illustrates that food is less defendable at lower densities, as indicated by reduced time spent on territorial defense. Obviously, food density in the second year was near the lower limit that would allow survival of rock pipits. When prey items are too sparse, encounters may be so infrequent that an individual cannot survive. Gibb (1960) calculated that to balance their energy budget during the winter in some places, English tits must find an insect on the average once every 2-1/2 seconds during daylight hours.
Time and energy budgets are influenced by a multitude of other ecological factors, including body size, mode of foraging, mode of locomotion, vagility, trophic level, prey size, resource density, environmental heterogeneity, rarefaction, competition, risks of predation, and reproductive tactics.
Leaf Tactics
Leaves take on an almost bewildering array of sizes and shapes: some leaves are deciduous, others evergreen; some are simple, others compound; and their actual spatial arrangement on a given plant differs considerably both within and between species (Figure 5.3).
 Figure 5.3. Leaves have evolved a spectacular variety of shapes and sizes, yet repeatable patterns do occur.
Figure 5.3. Leaves have evolved a spectacular variety of shapes and sizes, yet repeatable patterns do occur.
Some leaves are much more costly to produce and maintain than others (elementary economic considerations dictate that any given leaf must pay for itself plus generate a net energetic profit). Presumably, this great diversity of leaf tactics is a result of natural selection maximizing the lifetime reproductive success of individual plants under diverse environmental conditions. Leaf tactics are influenced by many factors that include light, water availability, prevailing winds, and herbivores. When grown in the shade, individuals of many species grow larger, less dissected leaves than when grown in the sun. Similarly, shade-tolerant plants of the understory usually have larger and less lobed leaves than canopy species. Similar types of leaves often evolve independently in different plant lineages subjected to comparable climatic conditions at different geographic localities, especially among trees (Bailey and Sinnot 1916; Ryder 1954; Stowe and Brown 1981). Compound leaves, thought to conserve woody tissue, with small leaflets are found in hot dry regions, whereas those with larger leaflets occur under warm moist conditions. Lowland wet tropical rain forest trees have large evergreen leaves with nonlobed or continuous margins, chaparral plants tend to have small sclerophyllous evergreen leaves, arid regions tend to support leafless stem succulents such as cacti or plants with entire leaf margins (especially among evergreens), plants from cold wet climates often have notched or lobed leaf margins, and so on. Such repeated patterns of leaf size and shape suggest that a general theory of leaf tactics is possible.
Several models for optimal leaf size under differing environmental conditions have been developed. Efficiency of water use (grams of carbon dioxide assimilated per gram of water lost) was the measure of plant performance maximized by Parkhurst and Loucks (1971). A similar model for size and shape of vine leaves was developed by Givnish and Vermeij (1976). Even these relatively simple models predict several observed patterns in leaf size, such as large leaves in warm, shady, wet places and small leaves in colder areas or warmer and sunnier locales (Figure 5.4).
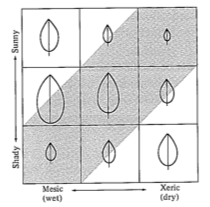 Figure 5.4. Patterns of leaf sizes predicted under differing environmental conditions from wet to dry (mesic to xeric) and shady to sunny. The shaded region represents conditions likely to prevail in nature. [From Givnish and Vermeij (1976). Copyright © 1976 by The University of Chicago Press.]
Figure 5.4. Patterns of leaf sizes predicted under differing environmental conditions from wet to dry (mesic to xeric) and shady to sunny. The shaded region represents conditions likely to prevail in nature. [From Givnish and Vermeij (1976). Copyright © 1976 by The University of Chicago Press.]
The evergreen versus deciduous dichotomy can be approached similarly using cost-benefit arguments (Orians and Solbrig 1977; Miller 1979). In considering leaf tactics of desert plants, Orians and Solbrig contrast leaf types along a continuum ranging from the relatively inexpensive deciduous "mesophytic" leaf to the more costly evergreen "xerophytic" leaf. Mesophytic leaves photosynthesize and transpire at a rapid rate and hence require high water availability (low "soil water potential"). In deserts, such plants grow primarily along washes. In contrast, xerophytic leaves cannot photosynthesize as rapidly when abundant water is available, but they can extract water from relatively dry soil. Each plant leaf tactic has an advantage either at different times or in different places, thereby promoting plant life form diversity. During wet periods, plants with mesophytic leaves photosynthesize rapidly, but under drought conditions, they must drop their leaves and become dormant. During such dry periods, however, the slower
photosynthesizers with xerophytic leaves are still able to function by virtue of their ability to extract water from dry soils. Of course, all degrees of intermediate leaf tactics exist, each of which may enjoy a competitive advantage under particular conditions of water availability (Figure 5.5). In a predictable environment, net annual profit per unit of leaf surface area determines the winning phenotype. Even a relatively brief wet season could suffice to give mesophytic leaves a higher annual profit (which accounts for the occurrence of these plant life forms in deserts).
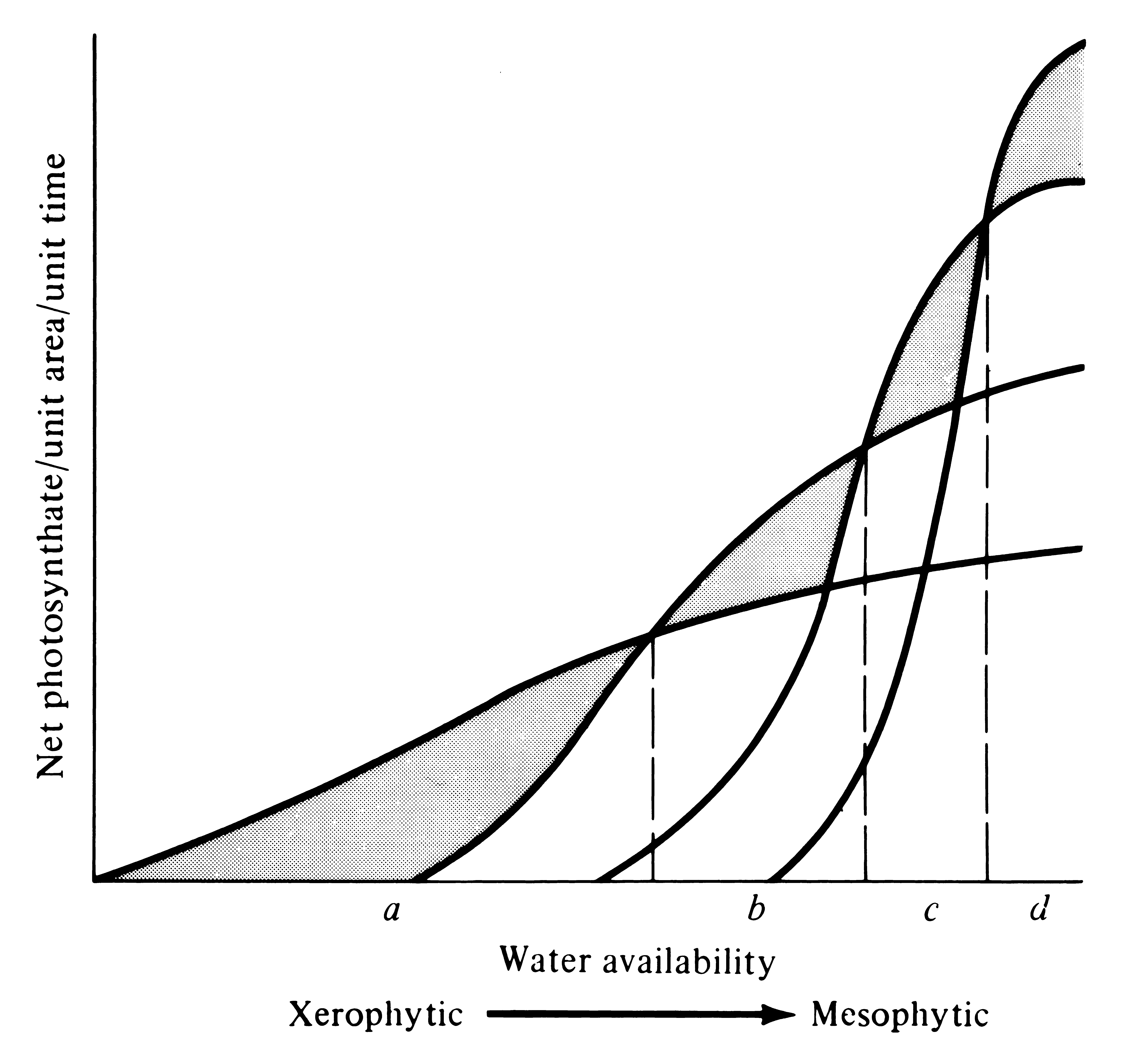 Figure 5.5. Probable relationship between efficiency of photosynthesis and water availability among different types of leaves. The most xerophytic leaf performs best under conditions of low water availability (zone a), whereas the most mesophytic leaf does best when soils are wet (zone d). Intermediate types are superior under intermediate conditions of water availability. Shaded areas indicate superiority of various leaf types under different conditions of soil moisture availability. [Adapted from Orians and Solbrig (1977). Copyright © 1977 by The University of Chicago Press.]
Figure 5.5. Probable relationship between efficiency of photosynthesis and water availability among different types of leaves. The most xerophytic leaf performs best under conditions of low water availability (zone a), whereas the most mesophytic leaf does best when soils are wet (zone d). Intermediate types are superior under intermediate conditions of water availability. Shaded areas indicate superiority of various leaf types under different conditions of soil moisture availability. [Adapted from Orians and Solbrig (1977). Copyright © 1977 by The University of Chicago Press.]
In an interesting discussion of leaf arrangement and forest structure, Horn (1971, 1975a, 1976) distinguished "monolayers" from "multilayers." Each plant in the multilayer of a forest (usually sunnier places such as the canopy) has leaves scattered throughout its volume at several different levels, whereas monolayer plants have essentially a single blanket or shell of leaves. Plants in the multilayer gain from a geometry that allows some light to pass through to their own leaves at lower levels. Horn points out that lobing facilitates passage of light and that such plants do well in the sun (in the shade, inner leaves may respire more than they photosynthesize). In contrast, the optimal tree design in the shade is a monolayer in which each leaf typically intercepts as much light as possible (leaves are large and seldom lobed). Moreover, slow-growing monolayered plants eventually outcompete fast-growing multilayered plants that persist by regular colonization of newly vacated areas created by continual disturbance (Horn 1976).
Foraging Tactics and Feeding Efficiency
Foraging tactics involve the ways in which animals gather matter and energy. As explained above, matter and energy constitute the profits gained from foraging, in that they are used in growth, maintenance, and reproduction. But foraging has its costs as well; a foraging animal may often expose itself to potential predators, and much of the time spent in foraging is rendered unavailable for other activities, including reproduction. An optimal foraging tactic maximizes the difference between foraging profits and their costs. Presumably, natural selection, acting as an efficiency expert, has often favored such optimal foraging behavior. Consider, for example, prey of different sizes and what might be termed "catchability." How great an effort should a foraging animal make to obtain a prey item with a given catchability and of a particular size (and therefore matter and energy content)? Clearly, an optimal consumer should be willing to expend more energy to find and capture food items that return the most energy per unit of expenditure upon them. Moreover, an optimal forager should take advantage of natural feeding routes and should not waste time and energy looking for prey either in inappropriate places or at inappropriate times. What is optimal in one environment is seldom optimal in another, and an animal's particular anatomy strongly constrains its optimal foraging tactic. Evidence is considerable that animals actually do attempt to maximize their foraging efficiencies, and a substantial body of theory on optimal foraging tactics exists.
Numerous aspects of optimal foraging theory are concisely summarized in an excellent chapter, "The Economics of Consumer Choice," by MacArthur (1972). He makes several preliminary assumptions: (a) Environmental structure is repeatable, with some statistical expectation of finding a particular resource (such as a habitat, microhabitat, and/or prey item). (b) Food items can be arranged in a continuous and unimodal spectrum, such as size distributions of insects (Schoener and Janzen 1968; Hespenhide 1971). (This assumption is clearly violated by foods of some animals, such as monophagous insects or herbivores generally, because plant chemical defenses are typically discrete; see Chapter 15) (c) Similar animal phenotypes are usually closely equivalent in their harvesting abilities; an intermediate phenotype is thus best able to exploit foods intermediate between those that are optimal for two neighboring phenotypes (see Chapter 13). Conversely, similar foods are gathered with similar efficiencies; a lizard with a jaw length that adapts it to exploit 5-mm-long insects best is only slightly less efficient at eating 4- and 6-mm insects. (d) The principle of allocation applies, and no one phenotype can be maximally efficient on all prey types; improving harvesting efficiency on one food type necessitates reducing the efficiency of exploiting other kinds of items. (e) Finally, an individual's economic "goal" is to maximize its total intake of food resources. (Assumptions b, c, and d are not vital to the argument.)
MacArthur (1972) then breaks foraging down into four phases: (1) deciding where to search; (2) searching for palatable food items; (3) upon locating a potential food item, deciding whether or not to pursue it; and (4) pursuit itself, with possible capture and eating. Search and pursuit efficiencies for each food type in each habitat are entirely determined by the preceding assumptions about morphology (assumption c) and environmental repeatability (assumption a); moreover, these efficiencies dictate the probabilities associated with the searching and pursuing phases of foraging (2 and 4, respectively). Thus, MacArthur considers only the two decisions: where to forage and what prey items to pursue (phases 1 and 3 of foraging).
Clearly, an optimal consumer should forage where its expectation of yield is greatest -- an easy decision to make, given knowledge of the previous efficiencies and the structure of its environment (in reality, of course, animals are far from omniscient and must make decisions based on incomplete information). The decision as to which prey items to pursue is also simple. Upon finding a potential prey item, a consumer has only two options: either pursue it or go on searching for a better item and pursue that one instead. Both decisions end in the forager beginning a new search, so the best choice is clearly the one that returns the greatest yield per unit time. Thus, an optimal consumer should opt to pursue an item only when it cannot expect to locate, catch, and eat a better item (i.e., one that returns more energy per unit of time) during the time required to capture and ingest the first prey item.
Many animals, such as foliage-gleaning insectivorous birds, spend much of their foraging time searching for prey but expend relatively little time and energy pursuing, capturing, and eating small sedentary insects that are usually easy to catch and quickly swallowed. In such "searchers," mean search time per item eaten is large compared to average pursuit time per item; hence, the optimal strategy is to eat essentially all palatable insects encountered. Other animals ("pursuers") that expend little energy in finding their prey but a great deal of effort in capturing it (such as, perhaps, a falcon or a lion) should select prey with small average pursuit times (and energetic costs). Hence, pursuers should generally be more selective and more specialized than searchers. Moreover, because a food-dense environment offers a lower average search time per item than does a food-sparse area, an optimal consumer should restrict its diet to only the better types of food items in the former habitat. To date, optimal foraging theory has been developed primarily in terms of the rate at which energy is gathered per unit of time. Limiting materials such as nutrients in short supply and the risks of predation have so far been largely neglected.
Carnivorous animals forage in extremely different ways. In the "sit-and-wait" mode, a predator waits in one place until a moving prey item comes by and then "ambushes" the prey; in the "widely foraging" mode, the predator actively searches out its prey (Pianka 1966b; Schoener 1969a, 1969b). The second strategy normally requires a greater energy expenditure than the first. The success of the sit-and-wait tactic usually depends on one or more of three conditions: a fairly high prey density, high prey mobility, and low predator energy requirements. The widely foraging tactic also depends on prey density and mobility and on the predator's energy needs, but here the distribution of prey in space and the predator's searching abilities assume paramount importance. Although these two tactics are endpoints of a continuum of possible foraging strategies (and hence somewhat artificial), foraging techniques actually employed by many organisms are rather strongly polarized. The dichotomy of sit-and-wait versus widely foraging therefore has substantial practical value. Among snakes, for example, racers and cobras forage widely when compared with boas, pythons, and vipers, which are relatively sit-and-wait foragers. Among hawks, accipiters such as Cooper's hawks and goshawks often hunt by ambush using a sit-and-wait strategy, whereas most buteos and many falcons are relatively more widely foraging. Web-building spiders and sessile filter feeders such as barnacles typically forage by sitting and waiting. Many spiders expend considerable amounts of energy and time building their webs rather than moving about in search of prey; those that do not build webs forage much more widely. Some general correlates of these two modes of foraging are listed in Table 5.1.
Table 5.1 Some General Correlates of Foraging Mode
_______________________________________________________________________
![]() Sit-and-Wait Sit-and-Wait![]() Widely Foraging Widely Foraging
_______________________________________________________________________
Prey type![]() Eat active prey Eat active prey![]() Eat sedentary and unpre- Eat sedentary and unpre-
![]() dictable (but clumped or dictable (but clumped or
![]() large) prey large) prey
Volume prey captured/day![]() Low Low![]() Generally high, but low Generally high, but low
![]() in certain species in certain species
Daily metabolic expense![]() Low Low![]() High High
Types of predators![]() Vulnerable primarily Vulnerable primarily![]() Vulnerable to both sit- Vulnerable to both sit-
![]() to widely foraging to widely foraging![]() and-wait and to widely and-wait and to widely
![]() predators predators![]() foraging predators foraging predators
Rate of encounters![]() Probably low Probably low![]() Probably high Probably high
with predators
Morphology![]() Stocky (short tails) Stocky (short tails)![]() Streamlined (generally Streamlined (generally
![]() long tails) long tails)
Probable physiological![]() Limited endurance Limited endurance![]() High endurance capacity High endurance capacity
correlates![]() (anaerobic) (anaerobic)![]() (aerobic) (aerobic)
Relative clutch mass![]() High High![]() Low Low
Sensory mode![]() Visual primarily Visual primarily![]() Visual or olfactory Visual or olfactory
Learning ability![]() Limited Limited![]() Enhanced learning and Enhanced learning and
![]() memory, larger brains memory, larger brains
Niche breadth![]() Wide Wide![]() Narrow Narrow
_______________________________________________________________________
Source: Adapted from Huey and Pianka (1981).
Similar considerations can be applied in comparing herbivores with carnivores.
Because the density of plant food almost always greatly exceeds the density of animal food, herbivores often expend little
energy, relative to carnivores, in finding their prey (to the extent that secondary chemical compounds of plants, such as
tannins, and other antiherbivore
defenses reduce palatability of plants or parts of plants, the effective supply of plant foods may be greatly reduced).
Because cellulose in plants is difficult to digest, however, herbivores must expend considerable energy in extracting
nutrients from their plant food. (Most herbivores have a large ratio of gut volume to body
volume, harbor intestinal microorganisms that digest cellulose, and spend much of their time eating or ruminating -- envision
a cow chewing its cud.) Animal food, composed of readily available proteins, lipids, and carbohydrates, is more readily digested;
carnivores can afford to expend considerable effort in searching for their prey because of the large dividends obtained once they
find it. As would be expected, the efficiency of conversion of food into an animal's own tissues
(assimilation) is considerably lower in herbivores than it is in carnivores.
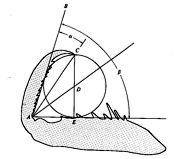 Figure 5.6. Diagram showing how Holling used geometry to calculate an estimated optimal size of a prey
item from the anatomy of a mantid's foreleg. Optimal prey diameter, D, is simply T sin (β - α),
where T is the distance A to C. [From Holling (1964).]
Figure 5.6. Diagram showing how Holling used geometry to calculate an estimated optimal size of a prey
item from the anatomy of a mantid's foreleg. Optimal prey diameter, D, is simply T sin (β - α),
where T is the distance A to C. [From Holling (1964).]
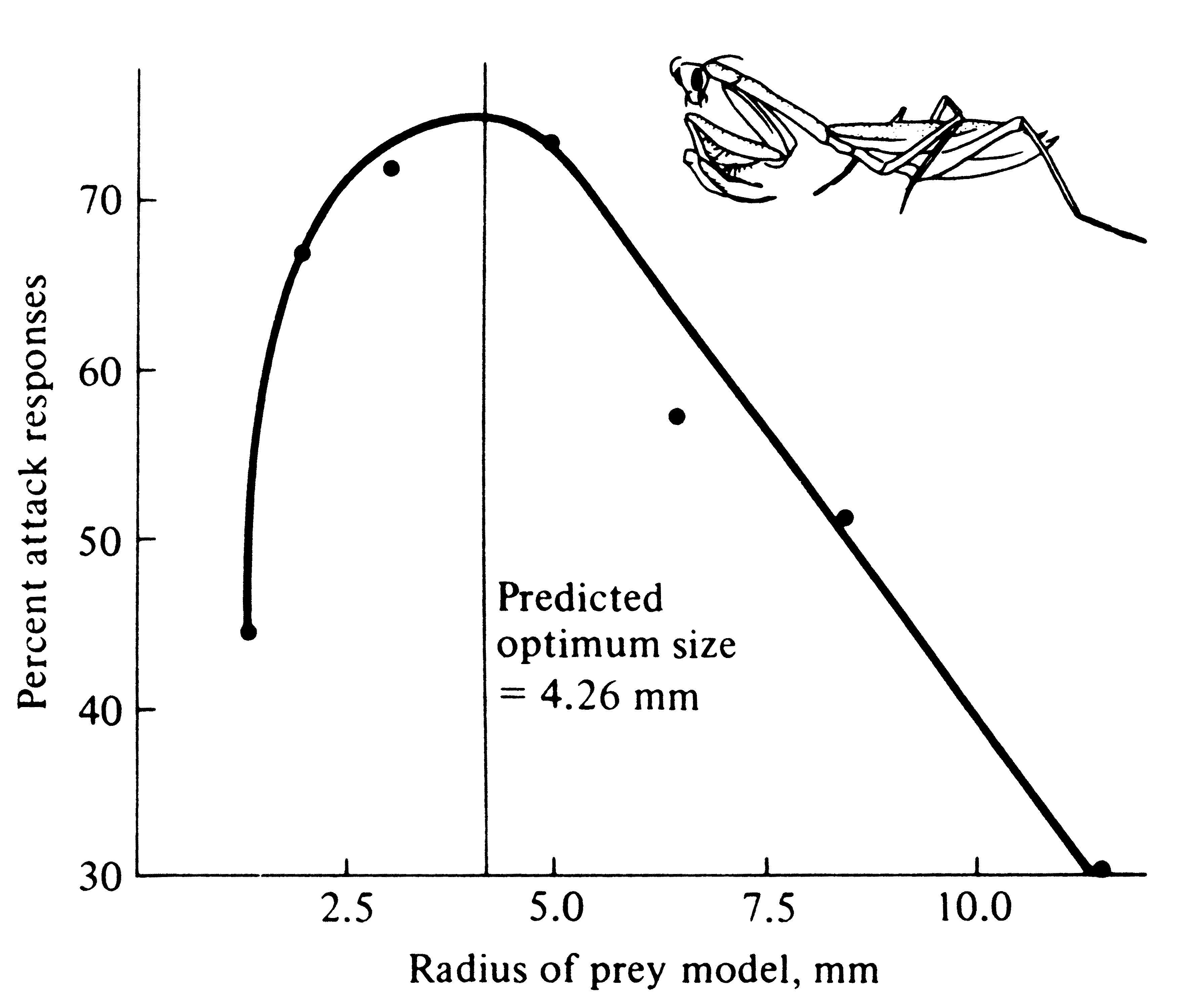 Figure 5.7. The percentage of prey items actually attacked
Figure 5.7. The percentage of prey items actually attacked
by hungry mantids versus prey size. [From Holling (1964).]
Many carnivores have extremely efficient prey-capturing devices (see Chapter 15); often the size of a prey object markedly influences this efficiency. Using simple geometry (Figure 5.6), Holling (1964) estimated the diameter of a prey item that should be optimal for a praying mantid of a particular size. He then offered hungry mantids prey objects of various sizes and recorded percentages attacked (Figure 5.7). Mantids were noticeably reluctant to attack prey that were either much larger or much smaller than the estimated optimum. Hence, natural selection has resulted in efficient predators both by producing efficient prey-capturing devices and by programming animals so that they are unlikely to attempt to capture decidedly suboptimal items. Larger predators tend to take larger prey than smaller ones (see Figure 12.12). It may in fact be better strategy for a large predator to overlook prey below some minimal size threshold and to spend the time that would have been spent in capturing and eating such small items in searching out larger prey (see also above). Similarly, the effort a predator will expend on any given prey item is proportional to the expected return from that item (which usually increases with prey size). Thus, a lizard waiting on a perch will not usually go far for a very small prey item but will often move much greater distances in attempts to obtain larger prey.
Because small prey are generally much more abundant than large prey, most animals encounter and eat many more small prey items than large ones. Small animals that eat small prey items encounter prey of suitable size much more frequently than do larger animals that rely on larger prey items; as a result, larger animals tend to eat a wider range of prey sizes. Because of such increased food niche breadths of larger animals, size differences between predators increase markedly with increasing predator size (MacArthur 1972).
Physiological Ecology
The concern of environmental physiology, or ecophysiology, is how organisms function within, adapt and respond to, and exploit their physical environments. Physiological ecologists are interested primarily in the immediate functional and behavioral mechanisms by which organisms cope with their abiotic environments. Physiological mechanisms clearly must reflect ecological conditions; moreover, mutual constraints between physiology and ecology dictate that both must evolve together in a synergistic fashion.
A fundamental principle of physiology is the notion of homeostasis, the maintenance of a relatively stable internal state under a much wider range of external environmental conditions. Homeostasis is achieved not only by physiological means but also by appropriate behavioral responses. An example is temperature regulation in which an organism maintains a fairly constant body temperature over a considerably greater range of ambient thermal conditions (homeostasis is never perfect). Homeostatic mechanisms have also evolved that buffer environmental variation in humidity, light intensity, and concentrations of various substances such as hydrogen ions (pH), salts, and so on. By effectively moderating spatial and temporal variation in the physical environment, homeostasis allows organisms to persist and be active within a broad range of environmental conditions, thereby enhancing their fitness. The subject of environmental physiology is vast; some references are given at the end of this chapter.
Physiological Optima and Tolerance Curves
Physiological processes proceed at different rates under different conditions. Most, such as rate of movement and photosynthesis, are temperature dependent (Figure 5.8). Other processes vary with availability of various materials such as water, carbon dioxide, nitrogen, and hydrogen ions (pH). Curves of performance, known as tolerance curves (Shelford 1913b), are typically bell shaped and unimodal, with their peaks representing optimal conditions for a particular physiological process and their tails reflecting the limits of tolerance. Some individuals and species have very narrow peaked tolerance curves; in others these curves are considerably broader. Broad tolerance curves are described with the prefix eury- (e.g., eurythermic, euryhaline), whereas steno- is used for narrow ones (e.g., stenophagous). An
organism's use of environmental resources such as foods and microhabitats can profitably be viewed similarly, and performance can be measured in a wide variety of units such as survivorship, reproductive success, foraging efficiency, and fitness.
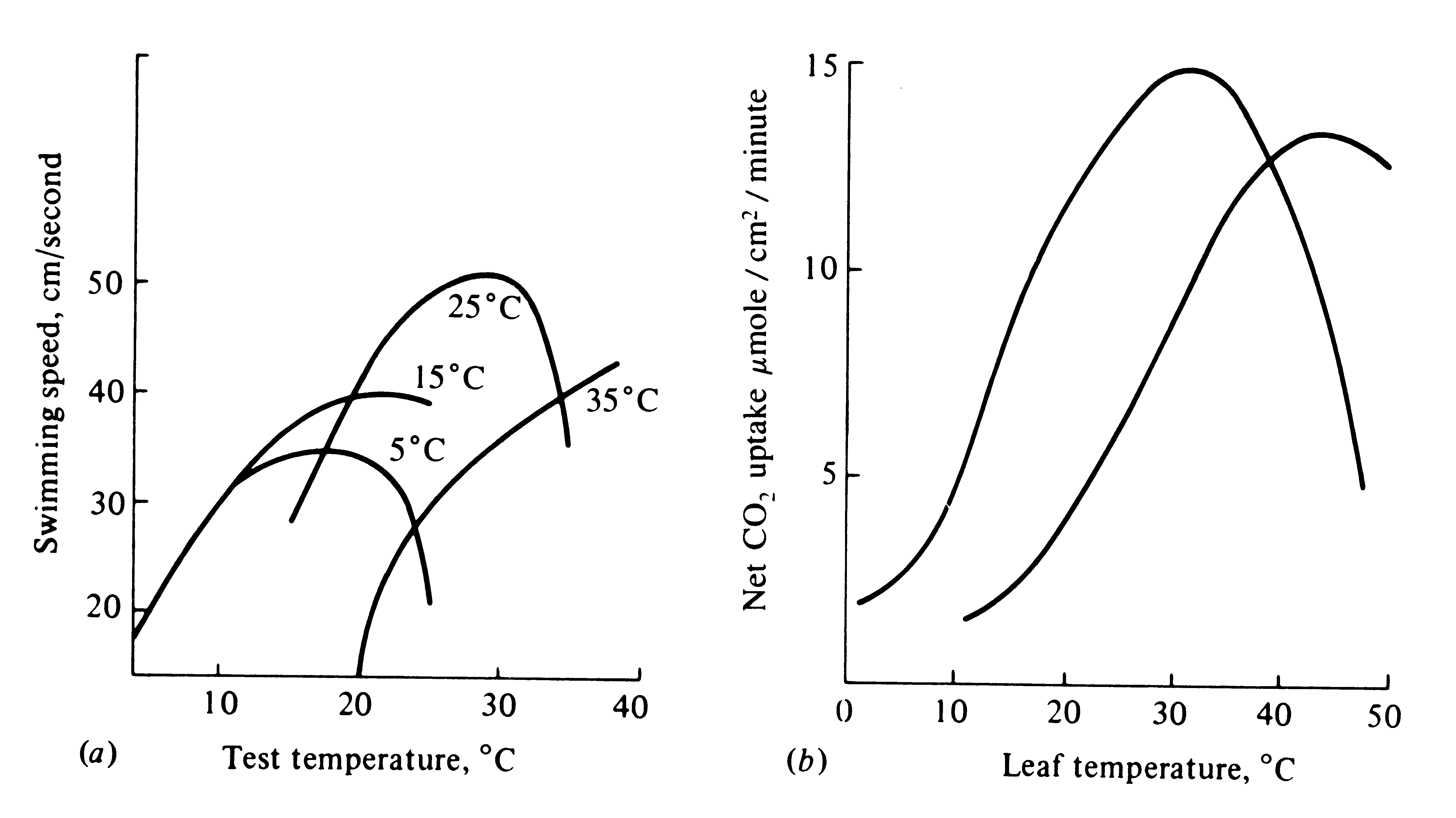 Figure 5.8. Two plots of performance against temperature. (a) Goldfish swimming speed versus temperature for fish acclimated to different thermal conditions; in most cases, performance peaks near the temperature to which fish are accustomed. [From Ricklefs (1973) after Fry and Hart (1948).] (b) Photosynthetic rate versus leaf temperature in the plant Atriplex lentiformis at two different localities. [From Mooney, Bjorkman, and Berry (1975).]
Figure 5.8. Two plots of performance against temperature. (a) Goldfish swimming speed versus temperature for fish acclimated to different thermal conditions; in most cases, performance peaks near the temperature to which fish are accustomed. [From Ricklefs (1973) after Fry and Hart (1948).] (b) Photosynthetic rate versus leaf temperature in the plant Atriplex lentiformis at two different localities. [From Mooney, Bjorkman, and Berry (1975).]
Performance curves can sometimes be altered during the lifetime of an
individual as it becomes exposed to different ambient external conditions. Such short-term alteration of
physiological optima is known as acclimation (Figure 5.8). Within certain design
constraints, tolerance curves clearly must change over evolutionary time as natural selection molds them to
reflect changing environmental conditions. However, very little is known about the evolution of tolerance;
most researchers have merely described the range(s) of conditions tolerated or exploited by particular
organisms. Tolerance curves are often taken almost as given and immutable, with little or no consideration
of the ecological and evolutionary forces that shape them.
Performance or tolerance is often sensitive to two or more
environmental variables. For example, the fitness of a hypothetical organism in various microhabitats
might be a function of relative humidity (or vapor pressure deficit), somewhat as shown in Figure 5.9a.
Assume that fitness varies similarly along a temperature gradient (Figure
5.9b).
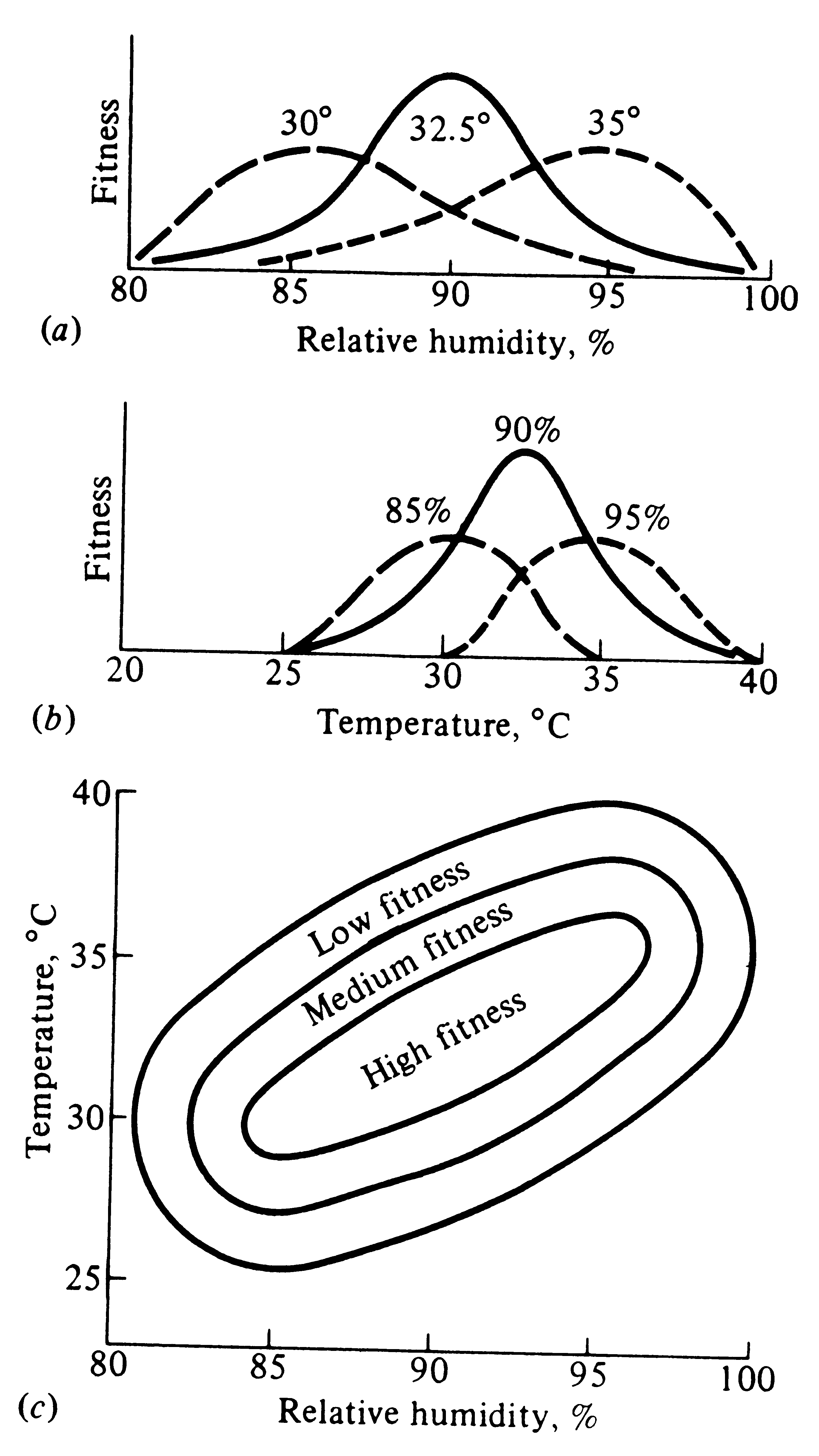 Figure 5.9c combines humidity and temperature conditions to show variation in fitness with respect
to both variables simultaneously (a third axis, fitness, is implicit in this figure). The range of thermal
conditions tolerated is narrower at very low and very high humidities than it is at intermediate and more
optimal humidities. Similarly, an organism's tolerance range for relative humidity is narrower at extreme
temperatures than it is at more optimal ones. The organism's thermal optimum depends on humidity conditions
(and vice versa). Fitness reaches its maximum at intermediate temperatures and humidities. Hence, temperature
tolerance and tolerance of relative humidities interact in this example. The concept of a single fixed optimum
is in some ways an artifact of considering only one environmental dimension at a time.
Figure 5.9c combines humidity and temperature conditions to show variation in fitness with respect
to both variables simultaneously (a third axis, fitness, is implicit in this figure). The range of thermal
conditions tolerated is narrower at very low and very high humidities than it is at intermediate and more
optimal humidities. Similarly, an organism's tolerance range for relative humidity is narrower at extreme
temperatures than it is at more optimal ones. The organism's thermal optimum depends on humidity conditions
(and vice versa). Fitness reaches its maximum at intermediate temperatures and humidities. Hence, temperature
tolerance and tolerance of relative humidities interact in this example. The concept of a single fixed optimum
is in some ways an artifact of considering only one environmental dimension at a time.
Figure 5.9. Hypothetical responses curves showing how two variables can interact
to determine an organism's fitness. Fitness is reduced at extremes of either temperature or humidity, and the
range of humidities tolerated is less at extreme temperatures than it is at intermediate ones.
Energetics of Metabolism and Movement
Some of the food ingested by any animal passes through its gut unused. Such egested material can be as high as 80 to 90 percent of the total intake in some caterpillars (Whittaker 1975). Food actually digested is termed assimilation: A fraction of this must be used in respiration to support maintenance metabolism and activity. The remainder is incorporated into the animal concerned as secondary productivity and ultimately can be used either in growth or in reproduction. These relationships are summarized below:
 Ingestion = Assimilation + Egestion Ingestion = Assimilation + Egestion
 Assimilation = Productivity + Respiration Assimilation = Productivity + Respiration
 Productivity = Growth + Reproduction Productivity = Growth + Reproduction
The total amount of energy needed per unit time for maintenance increases with increasing body mass (Figure 5.10). However, because small animals have relatively high ratios of body surface to body volume, they generally have much higher metabolic rates and hence have greater energy requirements per unit of body weight than larger animals (Figure 5.11). Animals that maintain relatively constant internal body temperatures are known as homeotherms; those whose temperatures vary widely from time to time, usually approximating the temperature of their immediate environment, are called poikilotherms. These two terms are sometimes confused with a related pair of useful terms. An organism that obtains its heat from its external environment is an ectotherm; one that produces most of its own heat internally by means of oxidative metabolism is known as an endotherm. All plants and the vast majority of animals are ectothermic; the only continuously endothermic animals are found among birds and mammals (but even some of these become ectothermic at times). Some poikilotherms (large reptiles and certain large fast-swimming fish such as tuna) are at times at least partially endothermic. Certain ectotherms (many lizards and temperate-zone flying insects) actually regulate their body temperatures fairly precisely during periods of activity by appropriate behavioral means. Thus, ectotherms at times can be homeotherms! An active bumblebee or desert lizard may have a body temperature as high as that of a bird or mammal (the layman's terms "warm-blooded" and "cold-blooded" can thus be quite misleading and should be abandoned). Because energy is required to maintain a constant internal body temperature, endotherms have considerably higher metabolic rates, as well as higher energy needs (and budgets), than ectotherms of
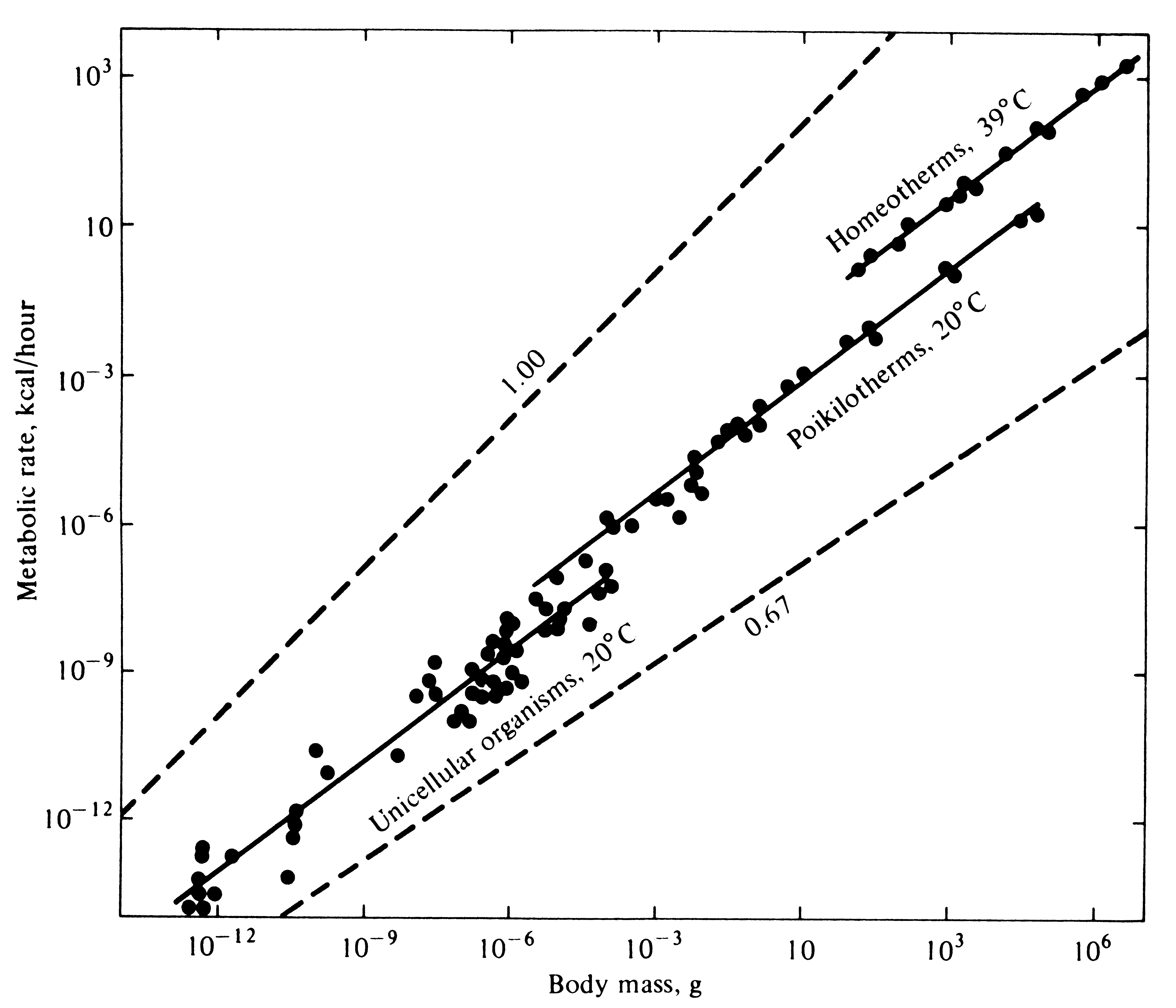 Figure 5.10. Metabolic rates of a variety of organisms of different sizes (log-log plot). Total oxygen consumption increases with increasing body size. [From Schmidt-Nielsen (1975).]
Figure 5.10. Metabolic rates of a variety of organisms of different sizes (log-log plot). Total oxygen consumption increases with increasing body size. [From Schmidt-Nielsen (1975).]
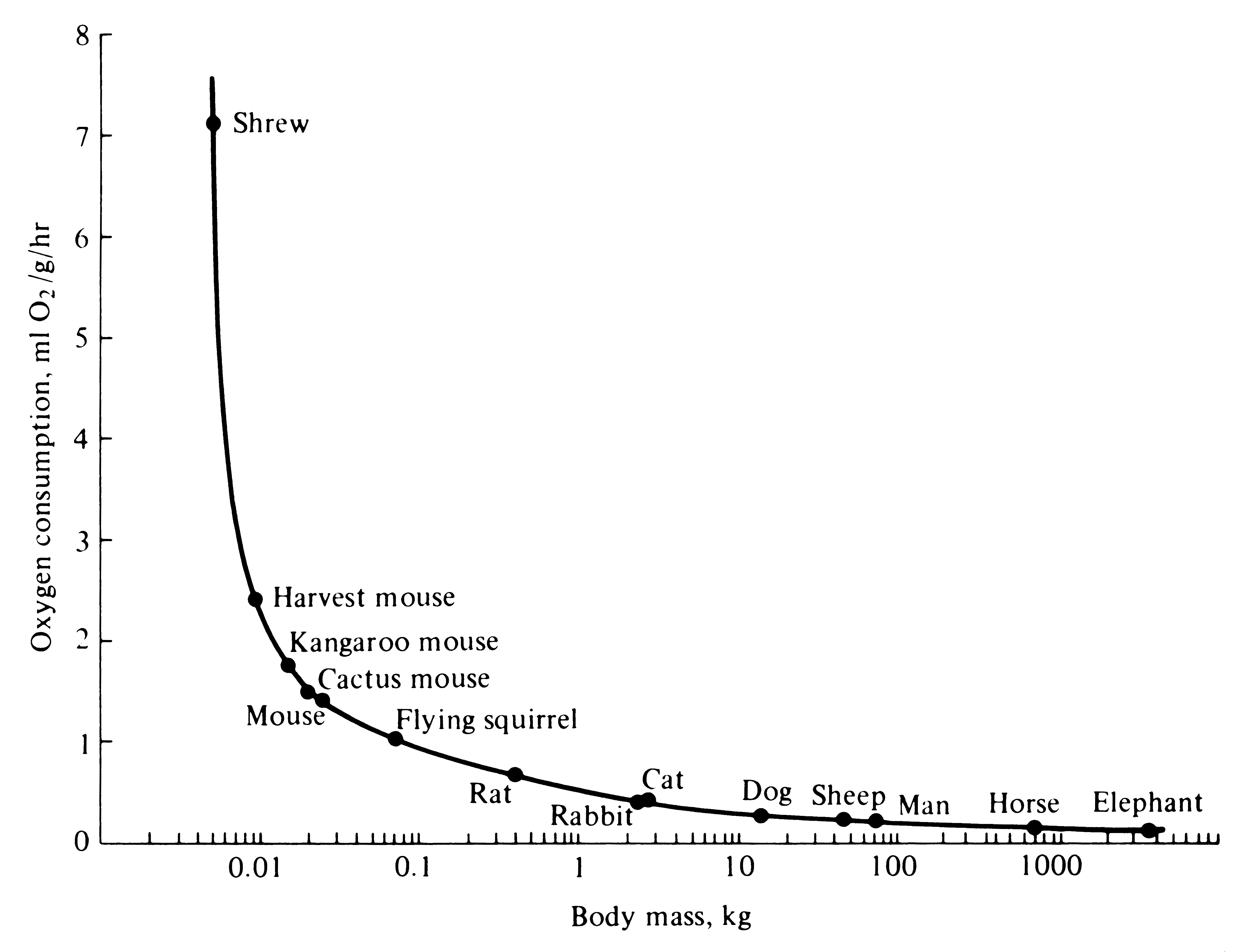 Figure 5.11. Semilogarithmic plot of rates of oxygen consumption per unit of body mass for a wide variety of mammals plotted against body mass. [From Schmidt-Nielsen (1975).]
Figure 5.11. Semilogarithmic plot of rates of oxygen consumption per unit of body mass for a wide variety of mammals plotted against body mass. [From Schmidt-Nielsen (1975).]
the same body mass. There is a distinct lower limit on body size for endotherms -- about the size of a small hummingbird or shrew (2 or 3 grams). Indeed, both hummingbirds and shrews have very high metabolic rates and hence rather precarious energetic relationships; they depend on continual supplies of energy-rich foods. Small hummingbirds would starve to death during cold nights if they did not allow their body temperatures to drop and go into a state of torpor.
Body size, diet, and movements are complexly intertwined with the energetics of metabolism. Energy requirements do not scale linearly with body mass, but instead as a fractional exponent: E = k m0.67 where k is a taxon-specific constant and m is body mass. Large animals require more matter and energy for their maintenance than small ones, and in order to obtain it they usually must range over larger geographical areas than smaller animals with otherwise similar food requirements. Food habits also influence movements and home range size. Because the foods of herbivorous animals that eat the green parts of plants (such as grazers, which eat grasses and ground-level vegetation, and browsers, which eat tree leaves) are usually quite dense, these animals usually do not have very large home ranges. In contrast, carnivores and those herbivores that must search for their foods (such as granivores and frugivores, which eat seeds and fruits, respectively) frequently spend much of their foraging time and energy in search, ranging over considerably larger areas. McNab (1963) termed the first group "croppers" and the latter "hunters." Croppers generally exploit foods that occur in relatively dense supply, whereas hunters typically utilize less dense foods. Hunters are often territorial, but croppers seldom defend territories. Croppers and hunters are not discrete but in fact grade into each other (Figure 5.12). A browser that eats only the leaves of a rare tree might be more of a hunter than a granivore that eats the seeds of a very common plant. However, such intermediates are uncommon enough that separation into two categories is useful for many purposes. Figure 5.13 shows the correlation between home range size and body weight for a variety of mammalian species, here separated into croppers and hunters. Analogous correlations, but with different slopes and/or intercepts, have been obtained for birds and lizards (Schoener 1968b; Turner et al. 1969). Very mobile animals, like birds, frequently range over larger areas than less mobile animals such as terrestrial mammals and lizards. In areas of low productivity (for example, deserts), most animals may be forced to range over a greater area to find adequate food than they would in more productive regions. Large home ranges or territories usually result in low densities, which in turn markedly limit possibilities for the evolution of sociality. Thus, McNab (1963) points out that complex social behavior has usually evolved only in croppers and/or among exceptionally mobile hunters.
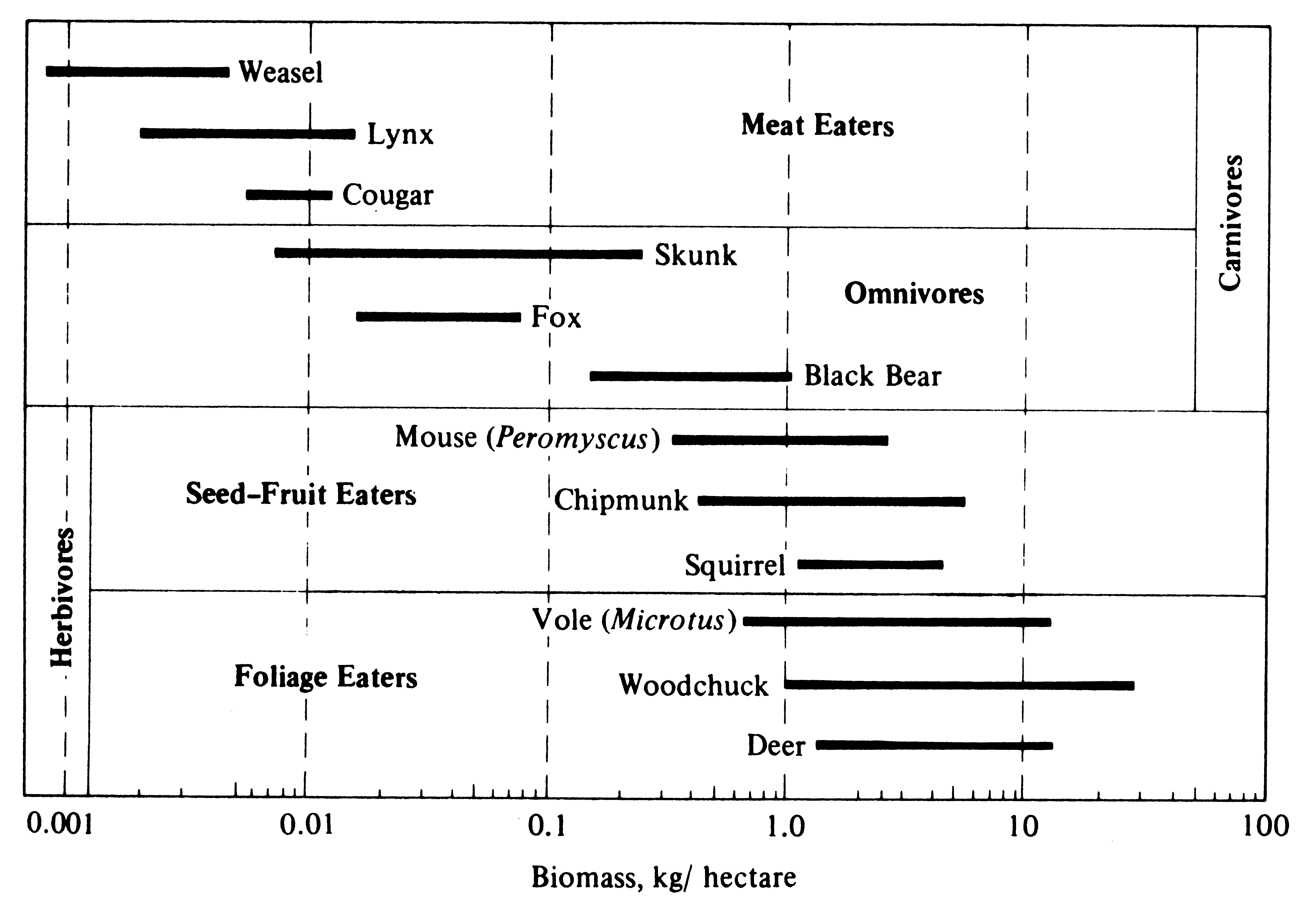 Figure 5.12. Biomass, in kilograms/hectare, of various mammals, arranged according to their food habits. Although mammals as a class vary over five orders of magnitude, the range among those that eat any given type of food is considerably smaller. Meat eaters and omnivores are much less dense than herbivores. [From Odum (1959) after Mohr (1940).]
Figure 5.12. Biomass, in kilograms/hectare, of various mammals, arranged according to their food habits. Although mammals as a class vary over five orders of magnitude, the range among those that eat any given type of food is considerably smaller. Meat eaters and omnivores are much less dense than herbivores. [From Odum (1959) after Mohr (1940).]
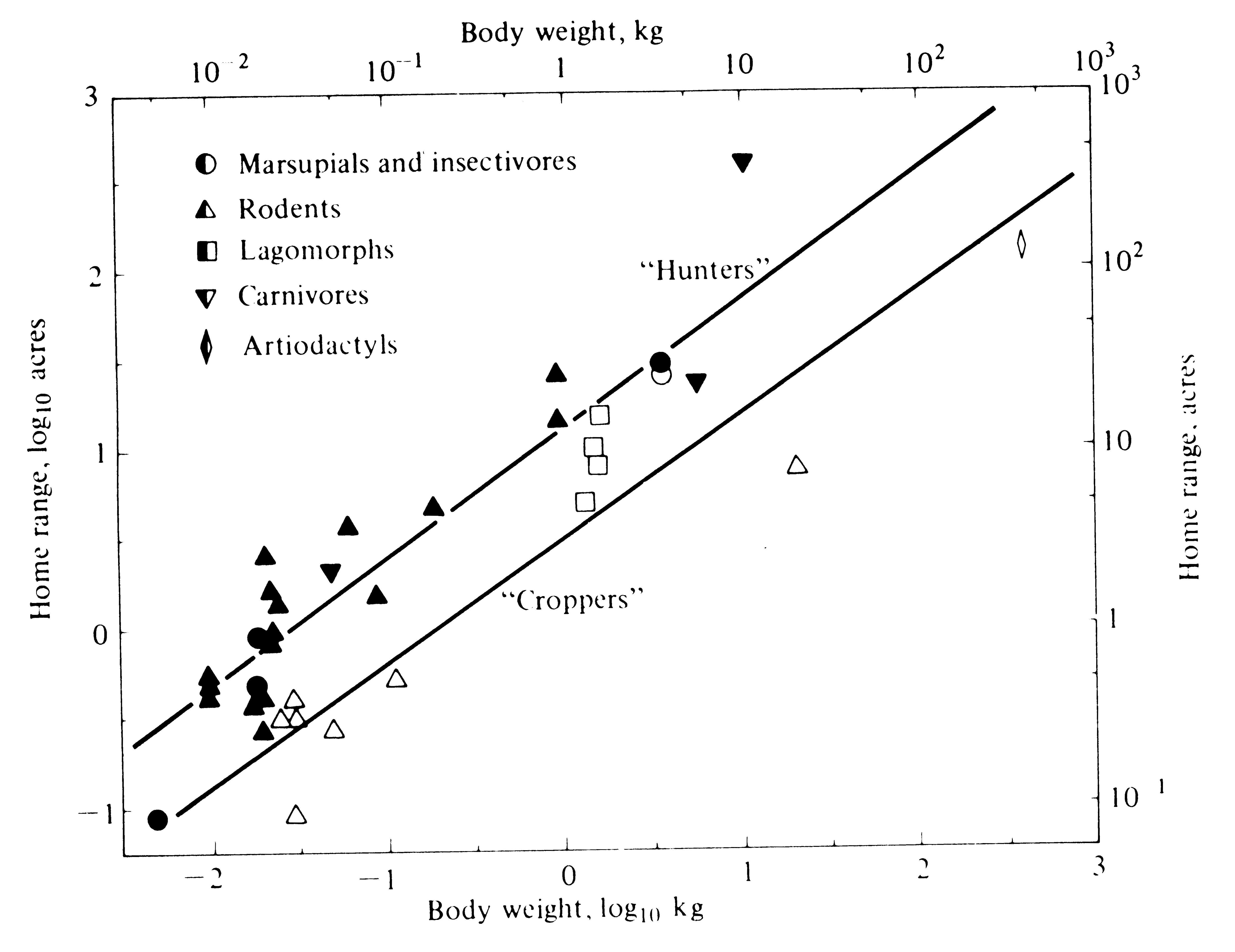 Figure 5.13. Log-log plot of average home range area against mean body mass for a variety of mammals, separated into "croppers" (open symbols and lower regression line) and "hunters" (closed symbols and upper line). [After McNab (1963).]
Figure 5.13. Log-log plot of average home range area against mean body mass for a variety of mammals, separated into "croppers" (open symbols and lower regression line) and "hunters" (closed symbols and upper line). [After McNab (1963).]
The metabolic cost of movement varies with both an animal's body size and its mode of locomotion. The cost of moving a unit of body mass some standard distance is actually less in larger animals than in smaller ones (Figure 5.14). Terrestrial locomotion is the most expensive mode of transportation, flight is intermediate in cost, and swimming is the most economical means of moving about -- provided body shape is fusiform and buoyancy is neutral (Figure 5.14).
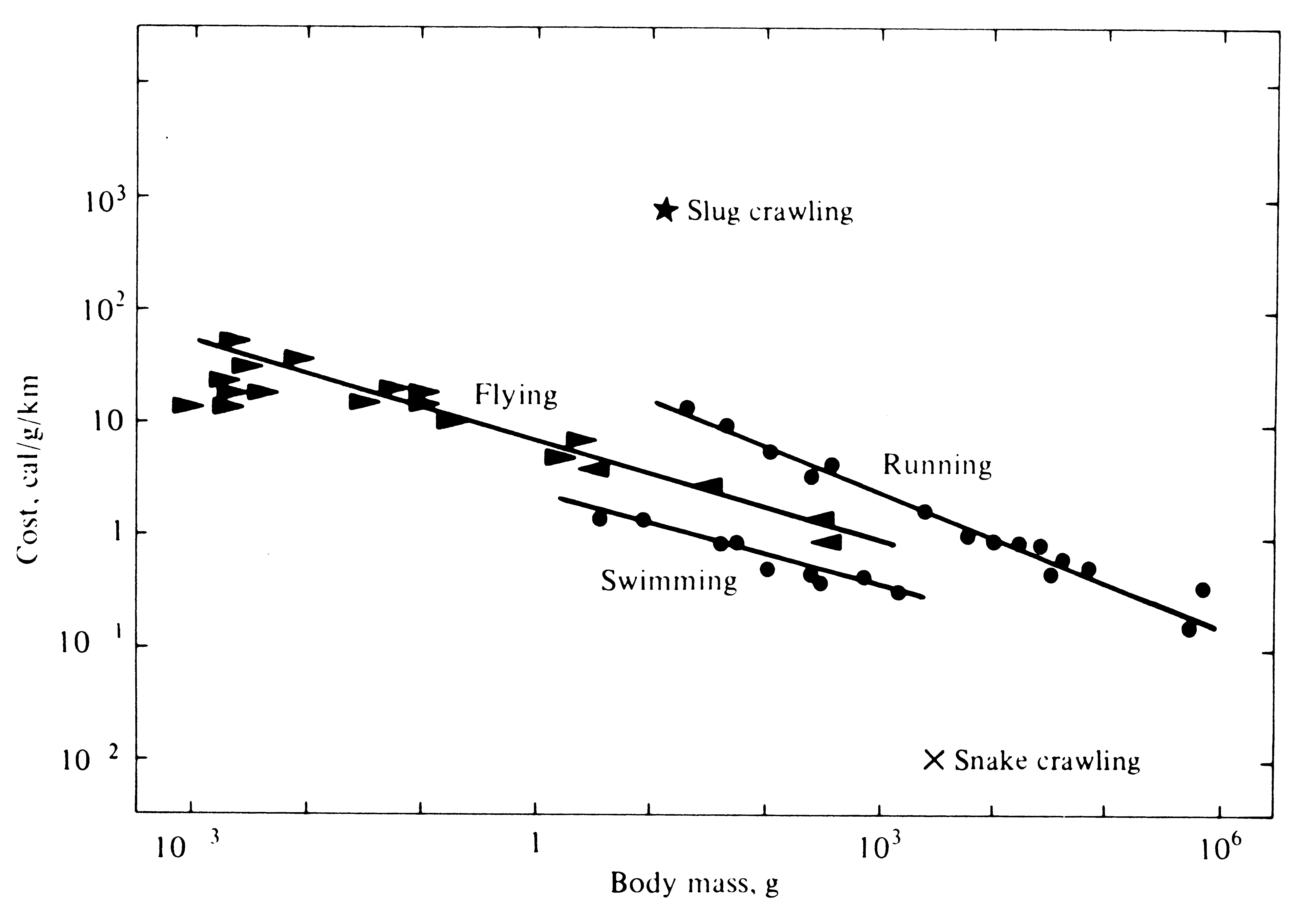 Figure 5.14. Comparison of the energetic cost of moving a unit of body mass one kilometer for several different modes of locomotion. [From Schmidt-Nielsen (1975).]
Figure 5.14. Comparison of the energetic cost of moving a unit of body mass one kilometer for several different modes of locomotion. [From Schmidt-Nielsen (1975).]
Physiologists have documented numerous consistent size-related trends in organs and metabolic properties. For example, among mammals, heart mass is always about 0.6 percent of total body mass, whereas blood volume is almost universally about 5.5 percent of body mass over a great range of body sizes (these organ systems are thus directly proportional to size). Other physiological attributes, such as lung surface in mammals, vary directly with metabolic rate rather than with size. However, some organ systems, such as the kidney and liver, do not scale directly with either size or metabolic rate (Schmidt-Nielsen 1975). Such "physiological rules" apparently dictate available avenues for physiological change, thereby constraining possible ecological adaptations.
Adaptation and Deterioration of Environment
Organisms are adapted to their environments in that, to survive and reproduce, they must meet their environment's conditions for existence. Evolutionary adaptation can be defined as conformity between an organism and its environment. Plants and animals have adapted to their environments both genetically and by means of physiological, behavioral, and/or developmental flexibility. The former includes instinctive behavior and the latter learning. Adaptation has many dimensions in that most organisms must conform simultaneously to numerous different aspects of their environments. Thus, for an organism to adapt, it must cope not only with various aspects of its physical environment, such as temperature and humidity conditions, but also with competitors, predators, and escape tactics of its prey. Conflicting demands of these various environmental components often require that an organism compromise in its adaptations to each. Conformity to any given component takes a certain amount of energy that is then no longer available for other adaptations. The presence of predators, for example, may require that an animal be wary, which in turn is likely to reduce its foraging efficiency and hence its competitive ability.
Organisms can conform to and cope with highly predictable environments relatively easily, even when they change in a regular way, as long as they are not too extreme. Adaptation to an unpredictable environment is usually more difficult; adapting to extremely erratic environments may even prove impossible. Many organisms have evolved dormant stages that allow them to survive unfavorable periods, both predictable and unpredictable. Annual plants everywhere and brine shrimp in deserts are good examples. Brine shrimp eggs survive for years in the salty crust of dry desert lakes; when a rare desert rain fills one of these lakes, the eggs hatch, the shrimp grow rapidly to adults, and they produce many eggs. Some seeds known to be many centuries old are still viable and have been germinated. Changes in the environment that reduce overall adaptation are collectively termed the "deterioration of environment"; such changes cause directional selection resulting in accommodation to the new environment.
A simple but elegant model of adaptation and undirected environmental deterioration was developed by Fisher (1930). He reasoned that no organism is "perfectly adapted" -- all must fail to conform to their environments in some ways and to differing degrees. However, a hypothetical, perfectly adapted organism can always be imagined (actually this reflects the environment) against which existing organisms may be compared. Fisher's mathematical argument is phrased in terms of an infinite number of "dimensions" for adaptation (only three are used here for ease of illustration).
Imagine an adaptational space of three coordinates representing, respectively, the competitive, predatory, and physical environments (Figure 5.15). An ideal "perfectly adapted" organism lies at a particular point (say A) in this space, but any given real organism is at another point (say B), some distance, d, away from the point of perfect adaptation. Changes in the position of A correspond to environmental changes making the optimally adapted organism different; changes in B represent changes in the organisms concerned, such as mutations. The distance between the two points, d, represents the degree of conformity between the organism and environment, or the level of adaptation. Fisher noted that very small undirected changes in either organism or environment have a 50:50 chance of being to the organism's advantage (i.e., reducing the distance between A and B). The probability of such improvement is inversely related to the magnitude of the change (Figure 5.16).
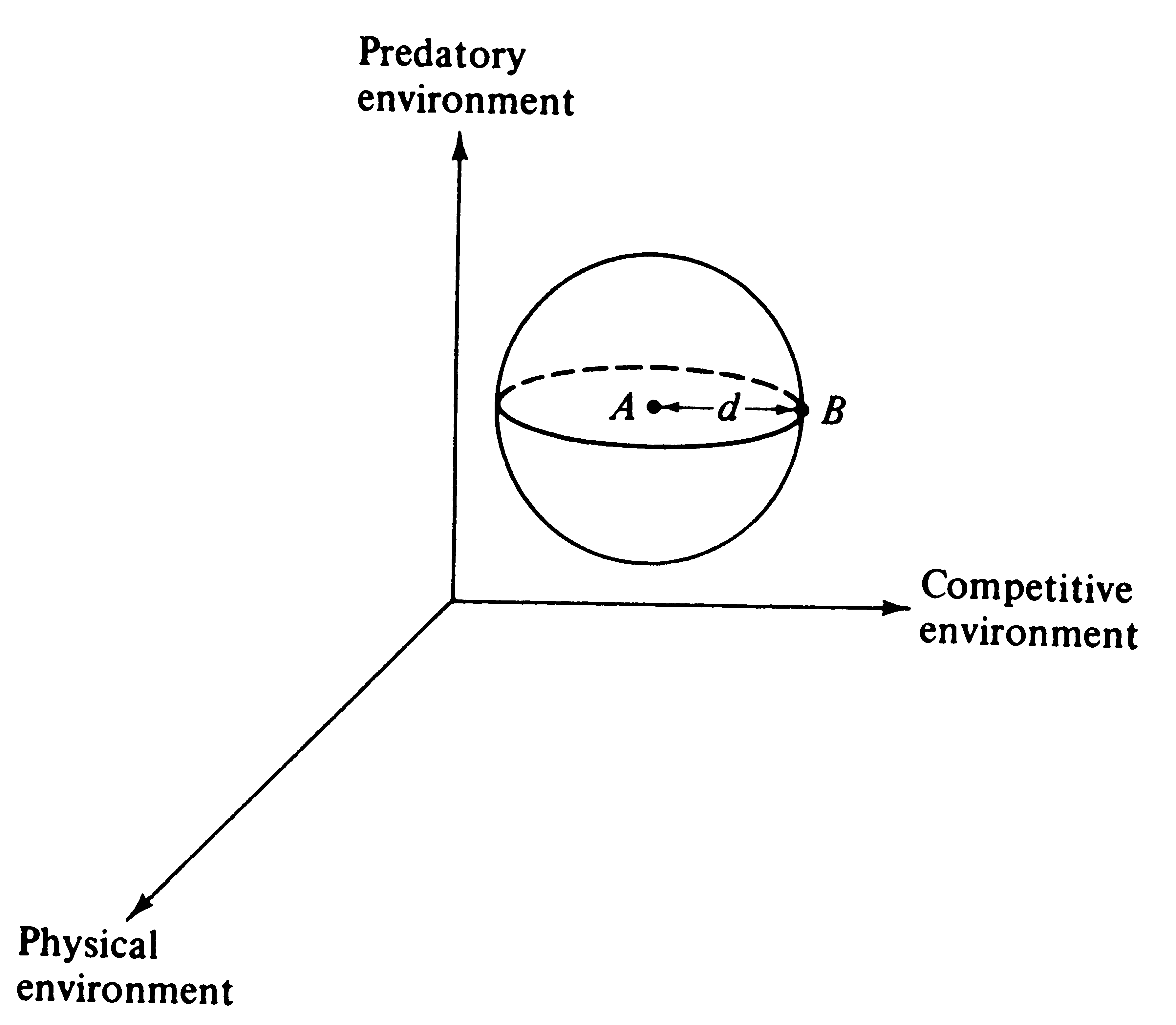 Figure 5.15. Fisher's model of adaptation and deterioration of environment. Point A represents a hypothetical "perfectly adapted" organism; an actual organism (point B) is never perfectly adapted and thus lies at some distance, d, from point A. The surface of the sphere represents all possible points with a level of adaptation equal to the organism under consideration. Very small undirected changes in either organism (point B) or environment (point A) are equally likely to increase or to decrease the level of adaptation, d. Notice the duality of the model (make A --> B and B --> A).
Figure 5.15. Fisher's model of adaptation and deterioration of environment. Point A represents a hypothetical "perfectly adapted" organism; an actual organism (point B) is never perfectly adapted and thus lies at some distance, d, from point A. The surface of the sphere represents all possible points with a level of adaptation equal to the organism under consideration. Very small undirected changes in either organism (point B) or environment (point A) are equally likely to increase or to decrease the level of adaptation, d. Notice the duality of the model (make A --> B and B --> A).
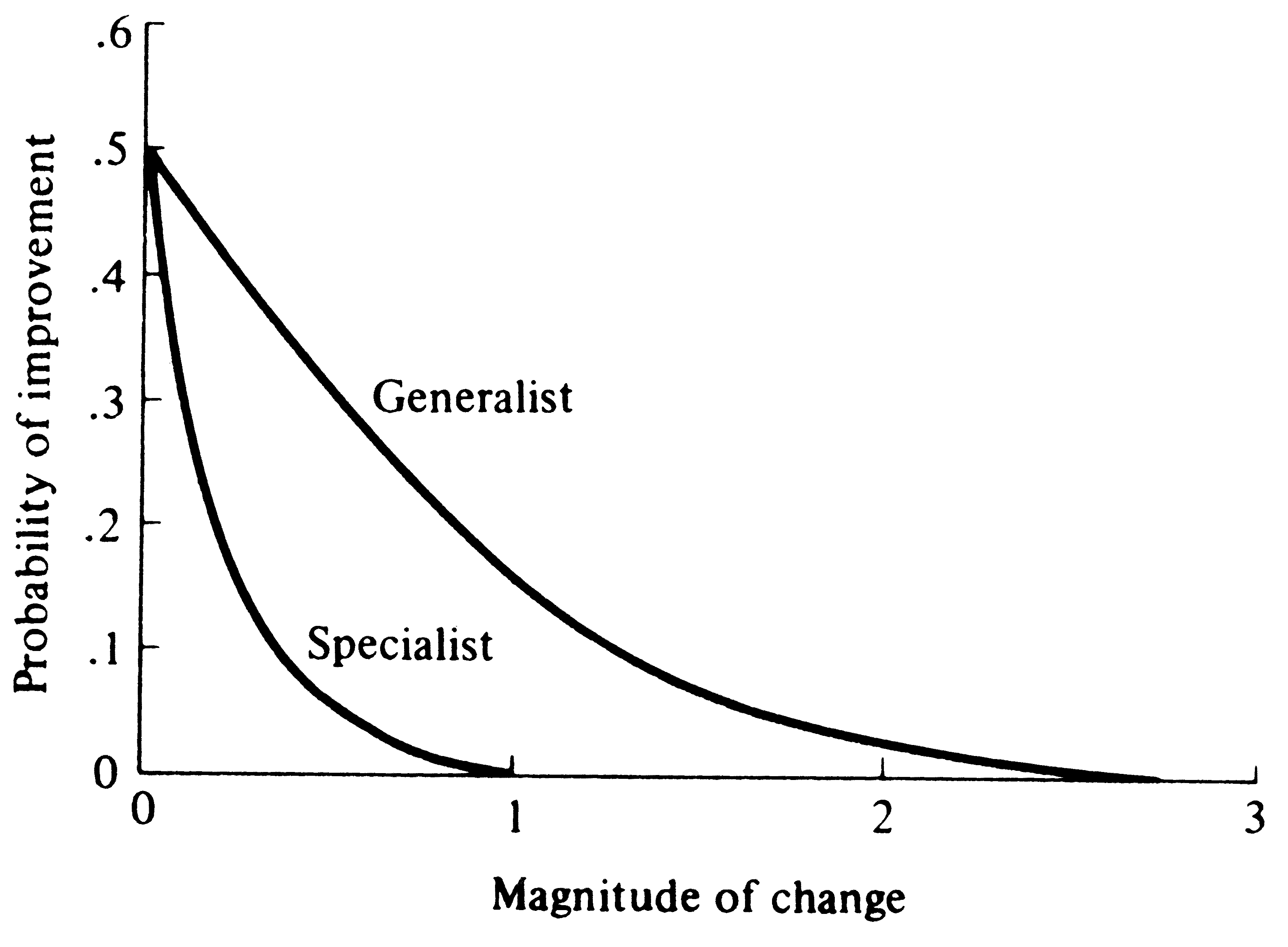 Figure 5.16. The probability of improvement of the level of adaptation (i.e., of reducing d) is plotted against the magnitude of an undirected change when the number of dimensions is large. Two hypothetical organisms are shown, one highly adapted such as a specialist with narrow tolerance limits and one less highly adapted such as a generalist with broader tolerance limits and/or a greater number of niche dimensions. A random change of a given magnitude is more likely to improve the level of adaptation of the generalist than the specialist. [Partially adapted from Fisher (1958a).]
Figure 5.16. The probability of improvement of the level of adaptation (i.e., of reducing d) is plotted against the magnitude of an undirected change when the number of dimensions is large. Two hypothetical organisms are shown, one highly adapted such as a specialist with narrow tolerance limits and one less highly adapted such as a generalist with broader tolerance limits and/or a greater number of niche dimensions. A random change of a given magnitude is more likely to improve the level of adaptation of the generalist than the specialist. [Partially adapted from Fisher (1958a).]
Very great changes in either organism or environment are always maladaptive because even if they are in the correct direction, they "overshoot" points of closer adaptation. (Of course, it is remotely possible that such major environmental changes or "macromutations" could put an organism into a completely new adaptive realm and thereby improve its overall level of adaptation.) Fisher makes an analogy with focusing a microscope. Very fine changes are as likely as not to improve the focus, but gross changes will almost invariably throw the machine further out of focus. Organisms may be thought of as "tracking" their environments in both ecological and evolutionary time, changing as their environments change; thus, as point A shifts because of daily, seasonal, and long-term environmental fluctuations, point B follows it. Such environmental tracking may be physiological (as in acclimation), behavioral (including learning), and/or genetic (evolutionary), depending on the time scale of environmental change. Reciprocal counterevolutionary responses to other species (prey, competitors, parasites, and predators) constitute examples of such evolutionary tracking, and have been termed coevolution (see Chapter 15). Individual organisms with narrow tolerance limits, such as highly adapted specialists, generally suffer greater losses in fitness due to a unit of environmental deterioration than generalized organisms with more versatile requirements, all else being equal. Thus, more specialized organisms and/or those with restricted homeostatic abilities cannot tolerate as much environmental change as generalists or organisms with better developed homeostasis (Figure 5.16). Fisher's (1930) model applies only to nondirected changes in either party of the adaptational complex -- such as mutations and perhaps certain climatic fluctuations, or other random events. However, many environmental changes are probably nonrandom. Changes in other associated organisms, especially predators and prey, are invariably directed so as to reduce an organism's degree of conformity to its environment; thus, they constitute a deterioration of that organism's environment. Directed changes in competitors can either increase or decrease an organism's level of adaptation, depending on whether they involve avoidance of competition or improvements in competitive ability per se. Directed changes in mutualistic systems would usually tend to improve the overall level of adaptation of both parties.
Heat Budgets and Thermal Ecology
When averaged over a long enough period of time, heat gained by an organism must be exactly balanced by heat lost to its environment; otherwise the plant or animal would either warm up or cool off. Many different pathways of heat gains and heat losses exist (Figure 5.17). The notion of a heat budget is closely related to the concept of an energy budget; balancing a heat budget requires very different adaptations under varying environmental conditions. At different times of day, ambient thermal conditions may change from being too cold to being too warm for a particular organism's optimal performance. Organisms living in hot deserts must avoid overheating by being able to minimize heat loads and to dissipate heat efficiently; in contrast, those that live in colder places such as at high altitudes or in polar regions must avoid overcooling -- hence they have evolved efficient means of heat retention, such as insulation by blubber, feathers, or fur, that reduce the rate of heat exchange with the external environment.
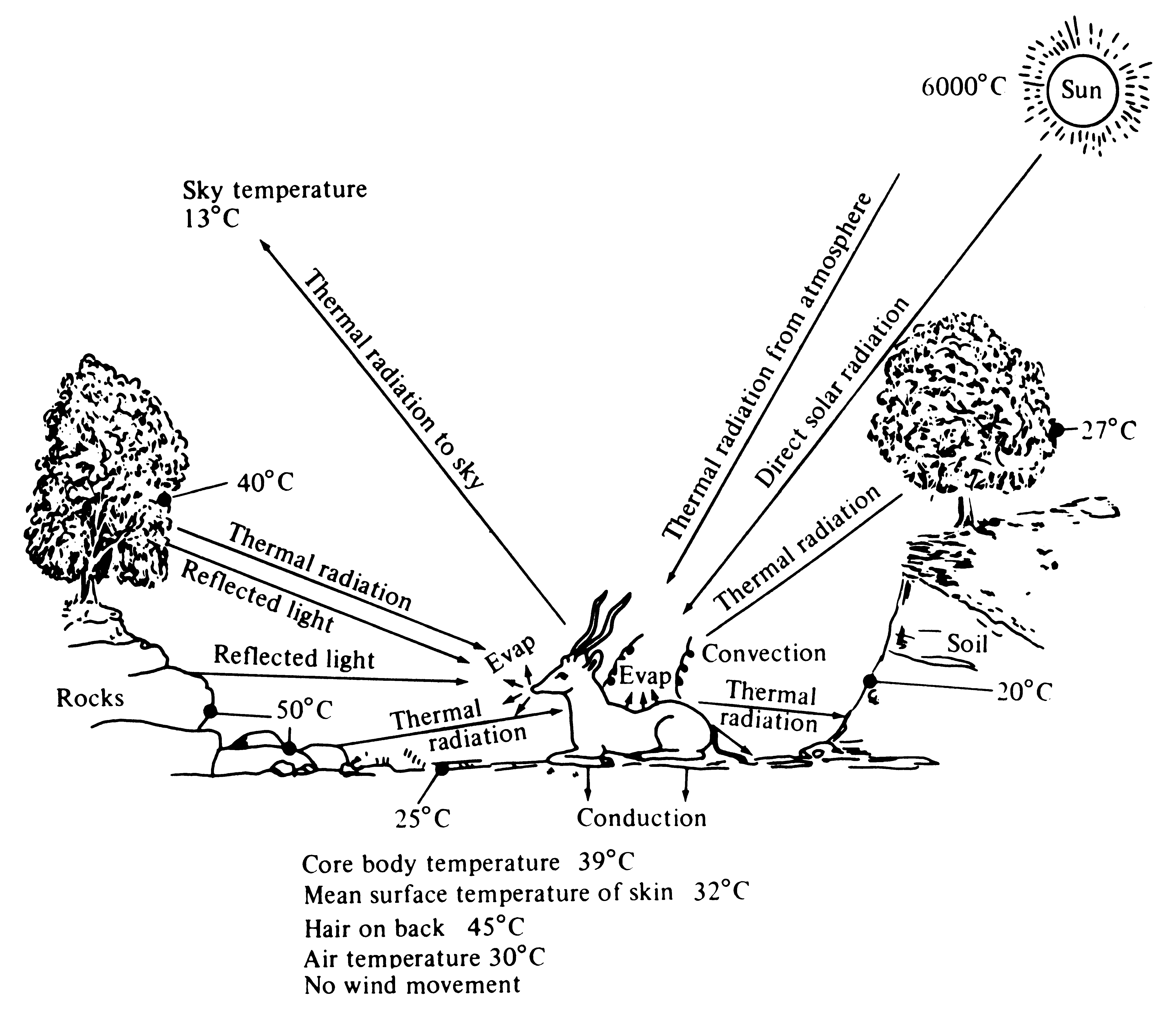 Figure 5.17. Diagrammatic representation of various pathways of heat gains and losses between an animal and its environment under moderately warm conditions. Heat exchange between an animal and its environment is roughly proportional to its body surface: Because small animals have a larger surface area relative to their weight than larger ones, the former gain or lose relatively more heat than the latter. [From Bartholomew (1972). Reprinted with permission of Macmillan Publishing Co., Inc., from Animal Physiology: Principles and Adaptations, M. S. Gordon (ed.). Copyright © 1972 by Malcolm S. Gordon.]
Figure 5.17. Diagrammatic representation of various pathways of heat gains and losses between an animal and its environment under moderately warm conditions. Heat exchange between an animal and its environment is roughly proportional to its body surface: Because small animals have a larger surface area relative to their weight than larger ones, the former gain or lose relatively more heat than the latter. [From Bartholomew (1972). Reprinted with permission of Macmillan Publishing Co., Inc., from Animal Physiology: Principles and Adaptations, M. S. Gordon (ed.). Copyright © 1972 by Malcolm S. Gordon.]
As seen in previous chapters, environmental temperatures fluctuate in characteristic ways at different places over the earth's surface, both daily and seasonally. In the absence of a long-term warming or cooling trend, environmental temperatures at any given spot remain roughly constant when averaged over an entire annual cycle. Recall that the range in temperature within a year is much greater at high latitudes than it is nearer the equator. An organism could balance its annual heat budget by being entirely passive and simply allowing its temperature to mirror that of its environment. Such a passive thermoregulator is known as a thermoconformer (Figures 5.18 and 5.19). Of course, it is also an ectotherm. Another extreme would be to maintain an absolutely constant body temperature by physiological and/or behavioral means, dissipating (or avoiding) excess bodily heat during warm periods but retaining (or gaining) heat during cooler periods (in endotherms, energy intake is often increased during cold periods and more metabolic heat is produced to offset the increased heat losses).
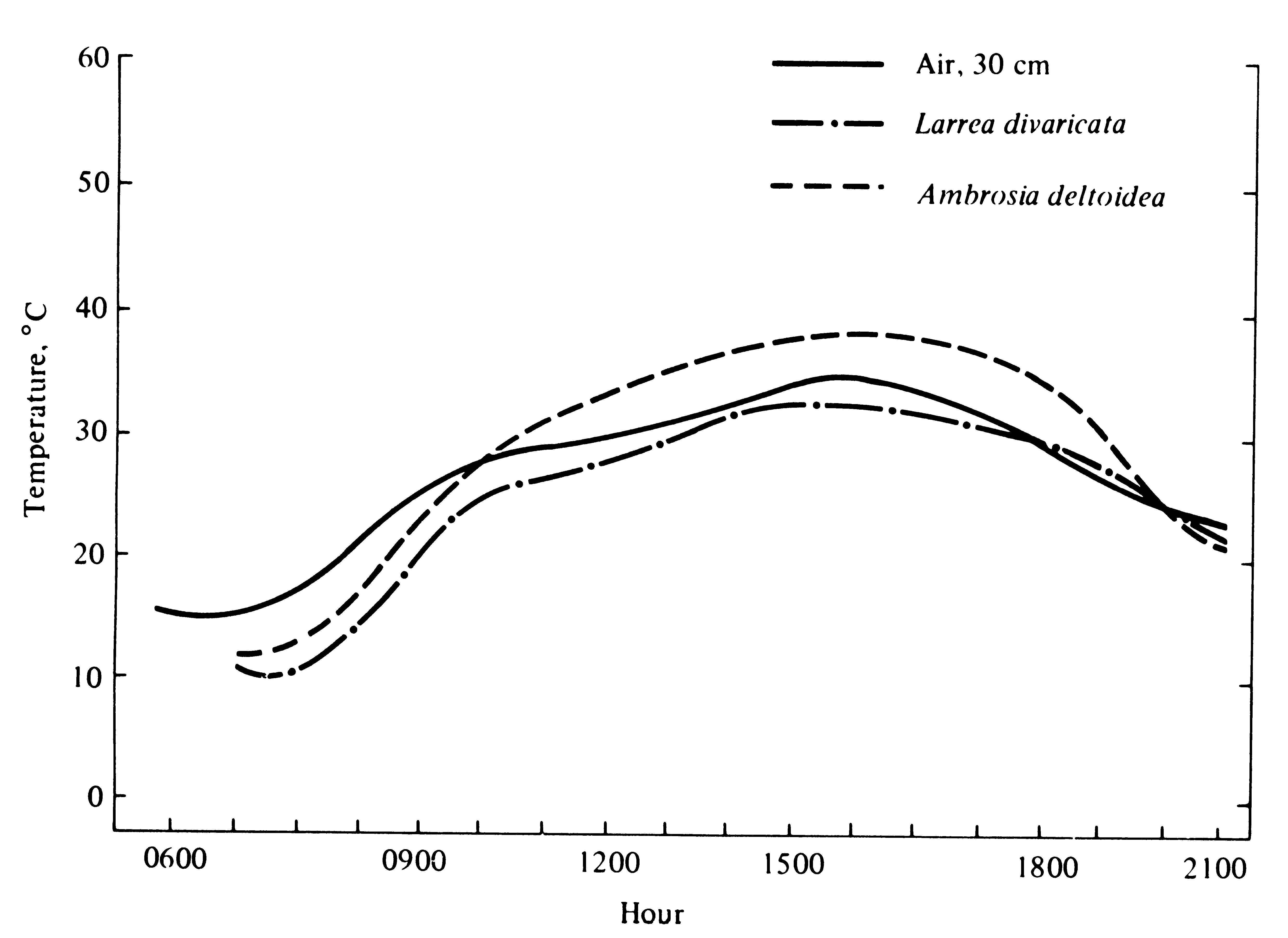 Figure 5.18. Average temperatures of leaves of Larrea divaricata and Ambrosia deltoidea during three sunny days in June with slight breezes prevailing. [From Patten and Smith (1975).]
Figure 5.18. Average temperatures of leaves of Larrea divaricata and Ambrosia deltoidea during three sunny days in June with slight breezes prevailing. [From Patten and Smith (1975).]
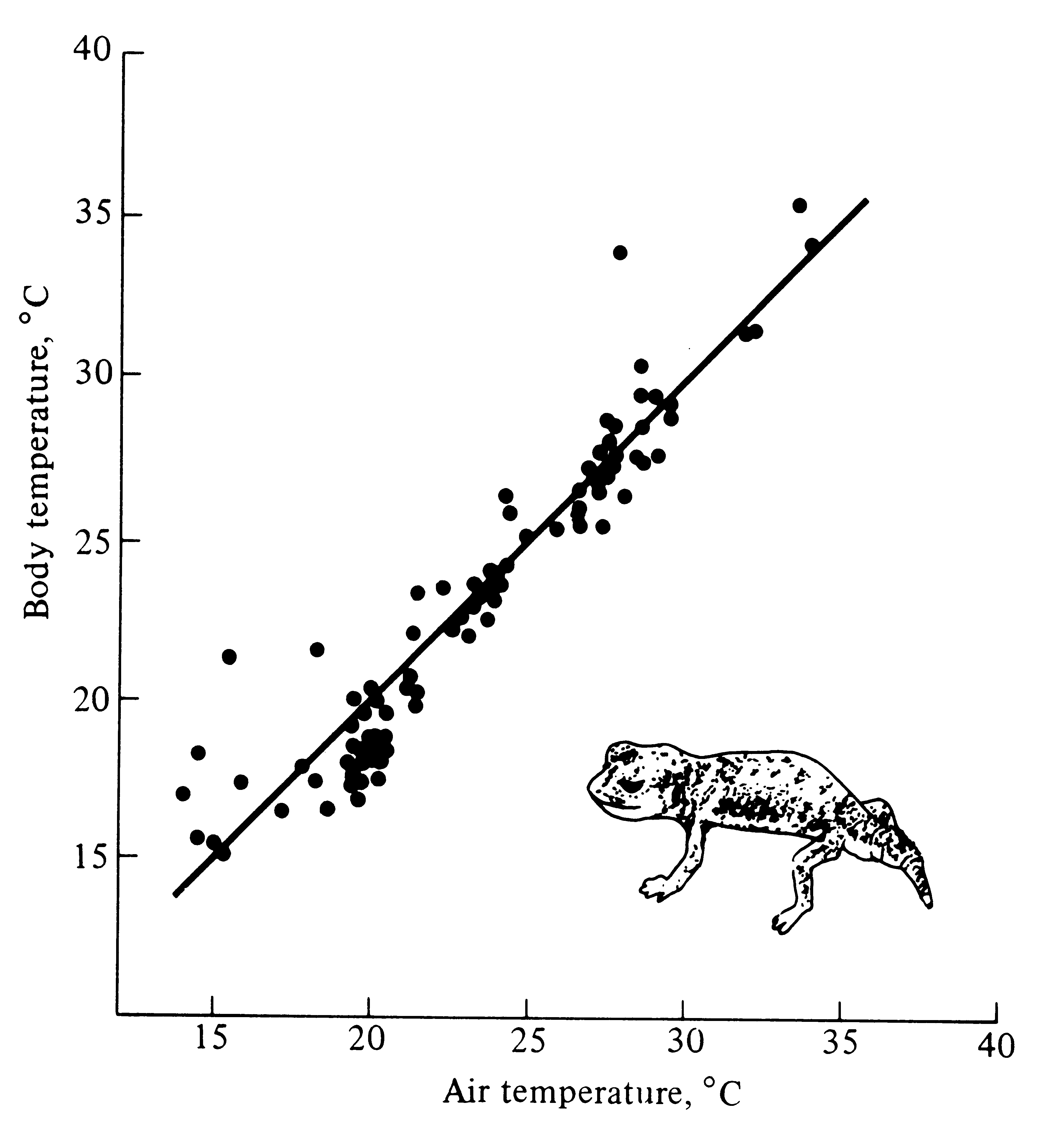 Figure 5.19. Body temperatures of 86 active Australian nocturnal gekkonid lizards (Nephrurus laevissimus) plotted against ambient air temperature. The line represents body temperatures equal to air temperatures. Like most nocturnal lizards, these animals are nearly perfect thermoconformers.
Figure 5.19. Body temperatures of 86 active Australian nocturnal gekkonid lizards (Nephrurus laevissimus) plotted against ambient air temperature. The line represents body temperatures equal to air temperatures. Like most nocturnal lizards, these animals are nearly perfect thermoconformers.
Organisms that carefully regulate their internal temperatures are called thermoregulators, or homeotherms. (Recall that both endotherms and ectotherms may regulate their body temperatures.) There is, of course, a continuum between the two extremes of perfect conformity and perfect regulation (see Figure 5.22). Homeostasis, remember, is never perfect. Because regulation clearly has costs and risks as well as profits, an emerging conceptual framework envisions an optimal level of regulation that depends on the precise form of the constraints and interactions among the costs and benefits arising from a particular ecological situation (Huey and Slatkin 1976). Thermoregulation often involves both physiological and behavioral adjustments; as an example of the latter, consider a typical terrestrial diurnal desert lizard. During the early morning, when ambient temperatures are low, such a lizard locates itself in warmer microclimates of the environmental thermal mosaic (e.g., small depressions in the open or on tree trunks),
basking in the sun with its body as perpendicular as possible to the sun's rays and thereby maximizing heat gained. With the daily march of temperature, ambient thermal conditions quickly rise and the lizard seeks cooler shady microhabitats. Individuals of some species retreat into burrows as temperatures rise; others climb up off the ground into cooler air and orient themselves facing into the sun's rays, thereby reducing heat load. Many lizards change colors and their heat reflectance properties, being dark and heat absorbent at colder times of day but light and heat reflectant at hotter times. Such adjustments allow individual lizards to be active over a longer period of time than they could be if they conformed passively to ambient thermal conditions; presumably, they are also more effective competitors and better able to elude predators as a result of such thermoregulatory behaviors.
Hot, arid regions typically support rich lizard faunas, whereas cooler forested areas have considerably fewer lizard species and individuals. Lizards can enjoy the benefits of a high metabolic rate during relatively brief periods when conditions are appropriate for activity and yet can still become inactive during adverse conditions. By facilitating metabolic inactivity on both a daily and a seasonal basis, poikilothermy thus allows lizards to capitalize on unpredictable food supplies. Ectotherms are low-energy animals; one day's food supply for a small bird will last a lizard of the same body mass for a full month! Most endothermic diurnal birds and mammals must wait out the hot midday period at considerable metabolic cost, whereas lizards can effectively reduce temporal heterogeneity by retreating underground, becoming inactive, and lowering their metabolic rate during harsh periods (some desert rodents estivate when food and/or water is in short supply). Poikilothermy may well contribute to the apparent relative success of lizards over birds and mammals in arid regions. Forests and grasslands are probably simply too shady and too cold for ectothermic lizards to be very successful because these animals depend on basking to reach body temperatures high enough for activity; in contrast, birds and mammals do quite well in such areas partly because of their endothermy.
Water Economy in Desert Organisms
Because water conservation is a major problem for desert organisms, their physiological and behavioral adaptations for acquisition of water and for economy of its use have been well studied. These interesting adaptations are quite varied. Like energy and heat budgets, water budgets must balance; losses must be replaced by gains. For examples of water acquisition mechanisms, consider rooting strategies. Desert plants may usually invest considerably more in root systems than plants from wetter areas; one study showed that perennial shrubs in the Great Basin desert allocate nearly 90 percent of their biomass to underground tissues (Caldwell and Fernandez 1975), whereas roots apparently represent a much smaller fraction (only about 10 percent) of the standing crop biomass of a mesic hardwood forest.
The creosote bush Larrea divaricata has both a surface root system and an extremely deep tap root that often reaches all the way down to the water table. This long tap root provides Larrea with water even during long dry spells when surface soils contain little moisture. Cacti, in contrast, have an extensive but relatively shallow root system and rely on water storage to survive drought. Many such desert plants have tough sclerophyllous xerophytic leaves that do not allow much water to escape (they also photosynthesize at a low rate as a consequence). Mesophytic plants occur in deserts too, but they photosynthesize rapidly and grow only during periods when moisture is relatively available; they drop their leaves and become inactive during droughts. Plants also reduce water losses during the heat of midday by closing their stomata and drooping their leaves (wilting). Many desert plants and animals absorb and use atmospheric and/or substrate moisture; most can also tolerate extreme desiccation.
Camels do not rely on water storage to survive water deprivation, as is commonly thought, but can lose as much as a quarter of their body mass, primarily as water loss (Schmidt-Nielsen 1964). Like many desert organisms, camels also conserve water by allowing their temperature to rise during midday. Moreover, camels tolerate greater changes in plasma electrolyte concentrations than less drought-adapted animals. In deserts, small mammals like kangaroo rats survive without drinking by relying on metabolic water derived from the oxidation of their food (here, then, is an interface between energy budgets and water budgets). Most desert rodents are nocturnal and avoid using valuable water for heat regulation by spending hot daytime hours underground in cool burrows with high relative humidity, thereby minimizing losses to evaporation (most desert organisms resort to evaporative cooling mechanisms such as panting only in emergencies). The urine of kangaroo rats is extremely concentrated and their feces contain little water (Schmidt-Nielsen 1964). Most other desert animals minimize water losses in excretion, too. Birds and lizards produce solid uric acid wastes rather than urea, thereby requiring little water for excretion. Desert lizards also conserve water by retreating to burrows and lowering their metabolic rate during the heat of the day.
Other Limiting Materials
Numerous other materials, including calcium, chloride, magnesium, nitrogen, potassium, and sodium, may be in short supply for particular organisms and must therefore be budgeted. Neural mechanisms of animals depend on sodium, potassium, and chloride ions, which are sometimes available in limited quantities. Because many herbivorous mammals obtain little sodium from their plant foods (plants lack nerves and sodium is not essential to their physiology), these animals must conserve sodium and/or find supplemental sources at salt licks -- indeed, Feeny (1975) suggests that plants may actually withhold sodium as an antiherbivore tactic. Similarly, amino acids are in short supply for many insects, such as in Heliconius butterflies, which supplement their diets with protein-rich pollen (Gilbert 1972).
An organism's nutrient and vitamin requirements are strongly influenced by the evolution of its metabolic pathways; likewise, these same pathways may themselves determine certain of the organism's nutritional needs. To illustrate: Almost all species of vertebrates synthesize their own ascorbic acid, but humans and several other primate species that have been tested cannot; they require a dietary supplement of ascorbic acid, known as vitamin C. Of thousands of other species of mammals, only two -- the guinea pig and an Indian fruit-eating bat -- are known to have lost the ability to synthesize their own ascorbic acid. A few species of birds must supplement their diets with ascorbic acid, too. Thus, a frog, a lizard, a sparrow, or a rat can make its own ascorbic acid, but we cannot. Why should natural selection favor the loss of the ability to produce a vital material? Pauling (1970) suggests that species of animals that have lost this capacity evolved in environments with ample supplies of ascorbic acid in available foods. It might actually be advantageous to dismantle a biochemical pathway in favor of another once it becomes redundant. Conversely, natural selection should favor evolution of the ability to synthesize any necessary materials that cannot be predictably obtained from available foods where this is possible (clearly organisms cannot synthesize elements -- herbivores cannot make sodium).
Sensory Capacities and Environmental Cues
Animals vary tremendously in their perceptive abilities. Most (except some cave dwellers and deep-sea forms) use light to perceive their environments. But visual spectra and acuity vary greatly. Some, such as insects, fish, lizards, and birds, have color vision, whereas others (most mammals, except squirrels and primates) do not. Ants, bees, and some birds can detect polarized light and exploit this ability to navigate by the sun's position; pigeons have poorly understood backup systems that enable them to return home remarkably well even when their vision is severely impaired with opaque contact lenses. Many temperate zone species of plants and animals rely on changes in day length to anticipate seasonal changes in climatic conditions. This, in turn, requires an accurate "biological clock." (Some organisms may also use barometric pressure to anticipate climatic changes.)
Certain snakes, such as pit vipers and boas, have evolved infrared receptors that allow them to locate and capture endothermic prey in total darkness. Most animals can hear, of course, although response to different frequencies varies considerably (some actually perceive ultrasonic sounds). Bats and porpoises emit and exploit sonar signals to navigate by echolocation. Similarly, nocturnal electric fish perceive their immediate environments by means of self-generated electrical fields. Certain bioluminescent organisms, such as "fireflies" (actually beetles), produce their own light for a variety of purposes, including attraction of mates and prey as well as (possibly) predator evasion (in some cases a mutualistic relationship is formed with a bioluminescent bacteria). Certain deep-sea fish probably use their "headlights" to find prey in the dark depths of the ocean. Pigeons can detect magnetic fields. Although a few animals have only a relatively feeble sense of smell (birds and humans, for example), most have keen chemoreceptors and/or olfactory abilities. Certain male moths can detect exceedingly dilute pheromones released by a female a full kilometer upwind, allowing these males to find females at considerable distances. Similarly, dung beetles use a zigzag flight to "home in" on upwind fecal material with remarkable precision.
Various environmental cues clearly provide particular animals with qualitatively and quantitatively different kinds and amounts of information. Certain environmental cues are useful in the context of capturing prey and escaping predators; others may facilitate timing of reproduction to coincide with good conditions for raising young. Some environmental signals are noisier and less reliable than others. Moreover, the ability to process information received from the environment is limited by a finite neural capacity. The principle of allocation and the notion of trade-offs dictate that an individual cannot perceive all environmental cues with high efficiency. If ability to perceive a broad range of environmental stimuli actually requires lowered levels of performance along each perceptual dimension, natural selection should improve perceptual abilities along certain critical dimensions at the expense of other less useful ones. Clearly, echolocation has tremendous utility for a nocturnal bat, whereas vision is relatively much less useful. In contrast, the values of these two senses are reversed for a diurnal arboreal squirrel. Within phylogenetic constraints imposed by its evolutionary history, an animal's sensory capacities can be viewed as a bioassay of the importance of particular perceptual dimensions and environmental cues in that animal's ecology.
Adaptive Suites
A basic point of this chapter is that any given organism possesses a unique coadapted complex of physiological, behavioral, and ecological traits whose functions complement one another and enhance that organism's reproductive success. Such a constellation of adaptations has been called an optimal design (Rosen 1967) or an adaptive suite (Bartholomew 1972).
Consider the desert horned lizard Phrynosoma platyrhinos (Figure 5.20). Various features of its anatomy, behavior, diet, temporal pattern of activity, thermoregulation, and reproductive tactics can be profitably interrelated and interpreted to provide an integrated view of the ecology of this interesting animal (Pianka and Parker 1975a). Horned lizards are ant specialists and usually eat essentially nothing else. Ants are small and contain much undigestible chitin, so that large numbers of them must be consumed. Hence, an ant specialist must possess a large stomach for its body size. When expressed as a proportion of total body mass, the stomach of this horned lizard occupies a considerably larger fraction of the animal's overall body mass (about 13 percent) than do the stomachs of all other sympatric desert lizard species, including the herbivorous desert iguana Dipsosaurus dorsalis (herbivores typically have lower assimilation rates and larger stomachs than carnivores). Possession of such a large gut necessitates a tanklike body form, reducing speed; selection has favored a spiny body form and cryptic behavior rather than a sleek body and rapid movement to cover (as in most other species of lizards), decreasing the lizard's ability to escape from predators by flight. As a result, natural selection has favored a spiny body form and cryptic behavior rather than a sleek body and rapid movement to cover (as in most other species of lizards).
Risks of predation are likely to be increased during long periods of exposure while foraging in the open. A reluctance to move, even when actually threatened by a potential predator, could well be
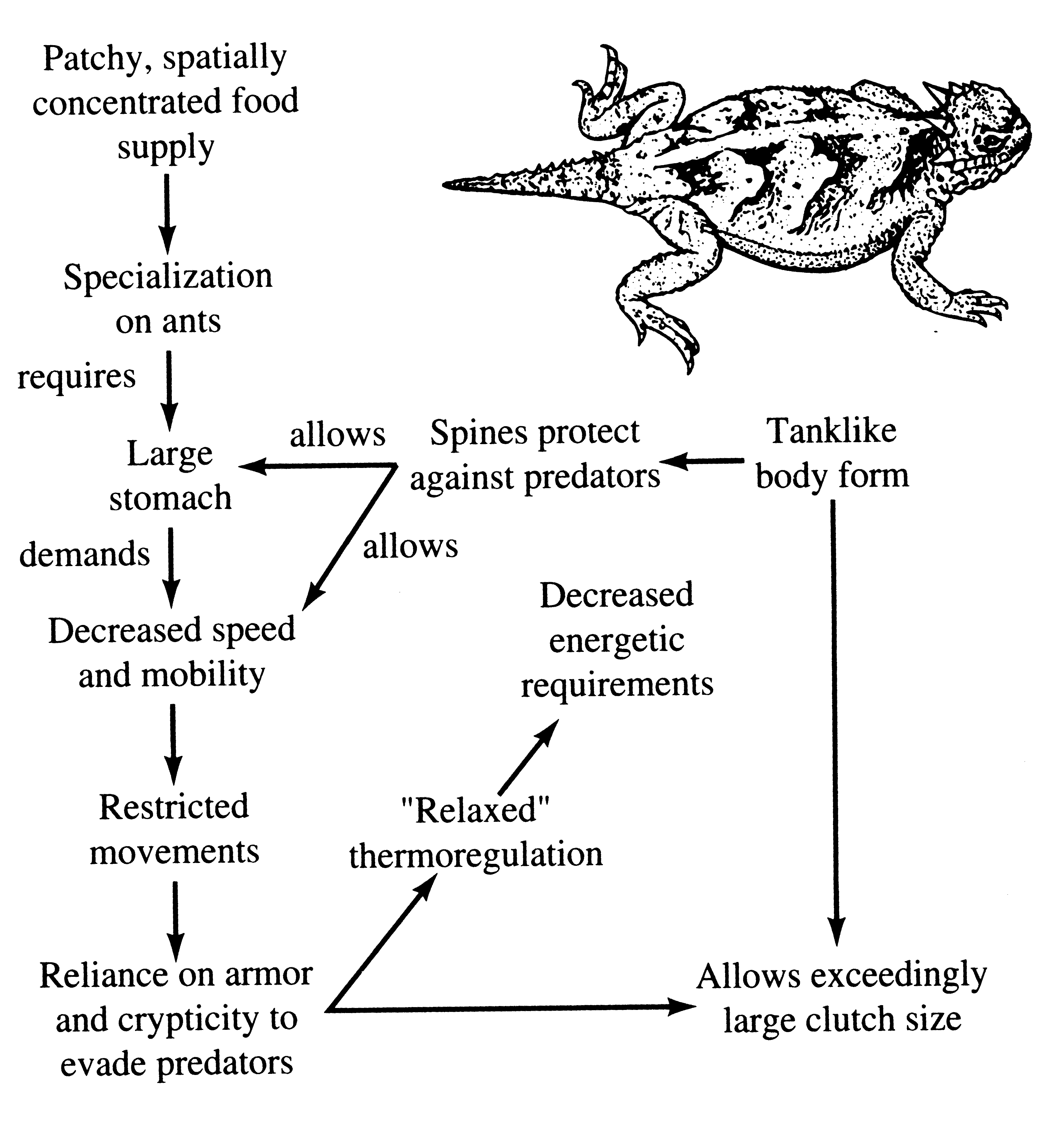 Figure 5.20. Diagrammatic portrayal of factors influencing the ecology and body form of the North American desert horned lizard, Phrynosoma platyrhinos.
Figure 5.20. Diagrammatic portrayal of factors influencing the ecology and body form of the North American desert horned lizard, Phrynosoma platyrhinos.
advantageous; movement might attract attention of predatorsand negate the advantage of concealing coloration and contour. Such decreased movement doubtless contributes to the observed high variance in body temperature of Phrynosoma platyrhinos, which is significantly greater than that of all other species of sympatric lizards.
Phrynosoma platyrhinos are also active over a longer time interval than any sympatric lizard species. Wide fluctuations in horned lizard body temperatures under natural conditions presumably reflect both the long activity period and perhaps their reduced movements into or out of the sun and shade (most of these lizards are in the open sun when first sighted). More time is thus made available for activities such as feeding. A foraging anteater must spend considerable time feeding. Food specialization on ants is economically feasible only because these insects usually occur in a clumped spatial distribution and hence constitute a concentrated food supply. To make use of this patchy and spatially concentrated, but at the same time not overly nutritious, food supply, P. platyrhinos has evolved a unique constellation of adaptations that include a large stomach, spiny body form, an expanded period of activity, and "relaxed" thermoregulation (eurythermy). The high reproductive investment of adult horned lizards is probably also a simple and direct consequence of their robust body form. Lizards that must be able to move rapidly to escape predators, such as racerunners (Aspidoscelis formerly Cnemidophorus), would hardly be expected to weight themselves down with eggs to the same extent as animals like horned lizards that rely almost entirely upon spines and camouflage to avoid their enemies.
Energetics of metabolism of weasels provide another, somewhat more physiological, example of a suite of adaptations (Brown and Lasiewski 1972). Due to their long, thin body shape, weasels have a higher surface-to-volume ratio than mammals with a more standard shape, and as a consequence, they have an increased energy requirement. Presumably, benefits of the elongate body form more than outweigh associated costs; otherwise natural selection would not have favored evolution of the weasel body shape. Brown and Lasiewski (1972) speculate that a major advantage of the elongate form is the ability to enter burrows of small mammals (weasel prey), which results in increased hunting success and thus allows weasels to balance their energy budgets (Figure 5.21). A further spin-off of the elongate shape is
 Figure 5.21. A schematic representation of factors involved in the evolution of elongate body shape in weasels. Circles indicate primary consequences of evolving a long, thin body configuration; ellipses show secondary consequences; rectangles indicate phenotypic characteristics of weasels affected by evolution of this body shape. Unbroken arrows indicate selective pressures and dashed arrows show causal sequences. Changes proceed in the direction of the arrows as long as selection favors a more elongate shape. [From Brown and Lasiewski (1972). Copyright © 1972 by the Ecological Society of America.]
Figure 5.21. A schematic representation of factors involved in the evolution of elongate body shape in weasels. Circles indicate primary consequences of evolving a long, thin body configuration; ellipses show secondary consequences; rectangles indicate phenotypic characteristics of weasels affected by evolution of this body shape. Unbroken arrows indicate selective pressures and dashed arrows show causal sequences. Changes proceed in the direction of the arrows as long as selection favors a more elongate shape. [From Brown and Lasiewski (1972). Copyright © 1972 by the Ecological Society of America.]
the evolution of a pronounced sexual dimorphism in body size, which allows male and female weasels to exploit prey of different sizes and hence reduces competition between the sexes (related mustelids such as skunks and badgers do not have the marked sexual size dimorphism characteristic of weasels).
Design Constraints
Most biologists are acutely aware that possible evolutionary pathways are somehow constrained by basic body plans. Although natural selection has "invented," developed, and refined a truly amazing variety of adaptations, selection is clearly far from omnipotent. Wheels might be a desirable solution to certain environmental contingencies and yet they have not been evolved. Such "design constraints" are usually elusive and not easily demonstrable. Students of thermoregulation have often noted an apparent upper thermal limit of about 40°C for most of the earth's eukaryotic creatures (most plants, invertebrates, and vertebrates). This thermal "lid" has frequently been used as evidence for some extremely archaic and inflexible fundamental physiological process (perhaps an enzyme basic to life processes, such as a dehydrogenase, denatures). The major exceptions are certain heat-tolerant bacteria and blue-green algae, inhabitants of hot springs and oceanic volcanic vents. These prokaryotic organisms may well have arisen before the origin of the heat-sensitive metabolic pathway that seems to limit the eukaryotes.
An example of such a physiological design constraint involves the thermal relationships of vertebrates, spanning classes from reptiles to mammals (Pianka 1985, 1986a). Detailed consideration of behavioral thermoregulation in lizards enables a fairly accurate prediction of the active body temperatures of mammalian homeotherms. A provocative biological "constant" can thus be identified that suggests a substantial degree of physiological inertia.
An intriguing hypothesis for the evolution of homeothermy was offered by Hamilton (1973), who suggested that homeothermy is a by-product of advantages gained from maintaining maximum body temperatures in the face of such an innate physiological ceiling. Ecologically optimal temperatures need not coincide with physiological optima.
Remember that not all homeotherms are endotherms; many ectotherms have attained a substantial degree of homeothermy by means of behavioral thermoregulation. Typically, such organisms actively select thermally suitable microhabitats, orient their bodies (or parts thereof) to control heat exchange, and/or shuttle between sun and shade as necessary to maintain a more-or-less constant internal body temperature.
Thermoregulation in lizards is not nearly as simple as it might appear to be at first glance, but rather encompasses a wide diversity of very different thermoregulatory tactics among species ranging from ectothermic poikilothermy to and including ectothermic homeothermy. Even a casual observer quickly notices that various species of desert lizards differ markedly in their times and places of activity. Some are active early in the morning, but other species do not emerge until late morning or midday. Most geckos and pygopodids and some Australian skinks are nocturnal. Certain species are climbers, others subterranean, while still others are strictly surface dwellers. Among the latter, some tend to be found in open areas whereas others frequent the edges of vegetation. Thermal relations of active lizards vary widely among species and are profoundly influenced by their spatial and temporal patterns of activity. Body temperatures of some diurnal heliothermic species average 38°C or higher, whereas those of nocturnal thigmothermic species are typically in the mid-twenties, closely paralleling ambient air temperatures.
Interesting interspecific differences also occur in the variance in body temperature as well as in the relationship between body temperatures and air temperatures. For example, among North American lizards, two arboreal species (Urosaurus graciosus and Sceloporus magister) display narrower variances in body temperature than do terrestrial species. Presumably, arboreal habits often facilitate efficient, economic, and rather precise thermoregulation. Climbing lizards have only to shift position slightly to be in the sun or shade or on a warmer or cooler substrate, and normally do not move through a diverse thermal environment. Moreover, arboreal lizards need not expend energy making long runs as do most ground dwellers, and thus climbing species do not raise their body temperatures metabolically to as great an extent as do terrestrial lizards.
Such differences in temporal patterns of activity, the use of space, and body temperature relationships are hardly independent. Rather, they complexly constrain one another, sometimes in intricate and obscure ways. For example, thermal conditions associated with particular microhabitats change in characteristic ways in time; a choice basking site at one time of day becomes an inhospitable hot spot at another time. Perches of arboreal lizards receive full sun early and late in the day when ambient air temperatures tend to be low and basking is therefore desirable, but these same tree trunks are shady and cool during the heat of midday when heat-avoidance behavior becomes necessary. In contrast, the fraction of the ground's surface in the sun is low when shadows are long early and late, but reaches a maximum at midday.
Terrestrial heliothermic lizards may thus experience a shortage of suitable basking sites early and late in the day; moreover, during the heat of the day, their movements through relatively extensive patches of open sun can be severely curtailed. Hence, ground-dwelling lizards encounter fundamentally different and more difficult thermal challenges than do climbing species.
Radiation and conduction are the most important means of heat exchange for the majority of diurnal lizards, although the thermal background in which these processes occur is strongly influenced by prevailing air temperatures. Ambient air temperatures are critical to nocturnal lizards as well as to certain very cryptic diurnal species.
In an analysis of the costs and benefits of lizard thermoregulatory strategies, Huey and Slatkin (1976) identified the slope of the regression of body temperature against ambient environmental temperature as a useful indicator (in this case, an inverse measure) of the degree of passiveness in regulation of body temperature. On such a plot of active body temperature versus ambient temperature, a slope of one indicates true poikilothermy or totally passive thermoconformity (a perfect correlation between air temperature and body temperature results), whereas a slope of zero reflects the other extreme of perfect thermoregulation. Lizards span this entire thermoregulation spectrum. Among active diurnal heliothermic species, regressions of body temperature on air temperature are fairly flat (for several species, including some quite small ones, slopes do not differ significantly from zero); among nocturnal species, slopes of similar plots are typically closer to unity. Various other species (nocturnal, diurnal, and crepuscular), particularly Australian ones, are intermediate, filling in this continuum of thermoregulatory tactics.
A straight line can be represented as a single point in the coordinates of slope versus intercept; these two parameters are plotted for linear regressions of body temperatures on air temperatures among some 82 species of lizards in Figure 5.22.
Each data point represents the least-squares linear regression of body temperature against air temperature for a given species of desert lizard. Interestingly enough, these data points fall on yet another, transcendent, straight line. The position of any
particular species along this spectrum reflects a great deal about its complex activities in space and time. The line plotted in Figure 5.22 thus offers a potent linear dimension on which various species can be placed in attempts to formulate general schemes of lizard ecology (Pianka 1985, 1986a, 1993). Various other ecological parameters, including reproductive tactics, can be mapped on to this emergent spatial temporal axis.
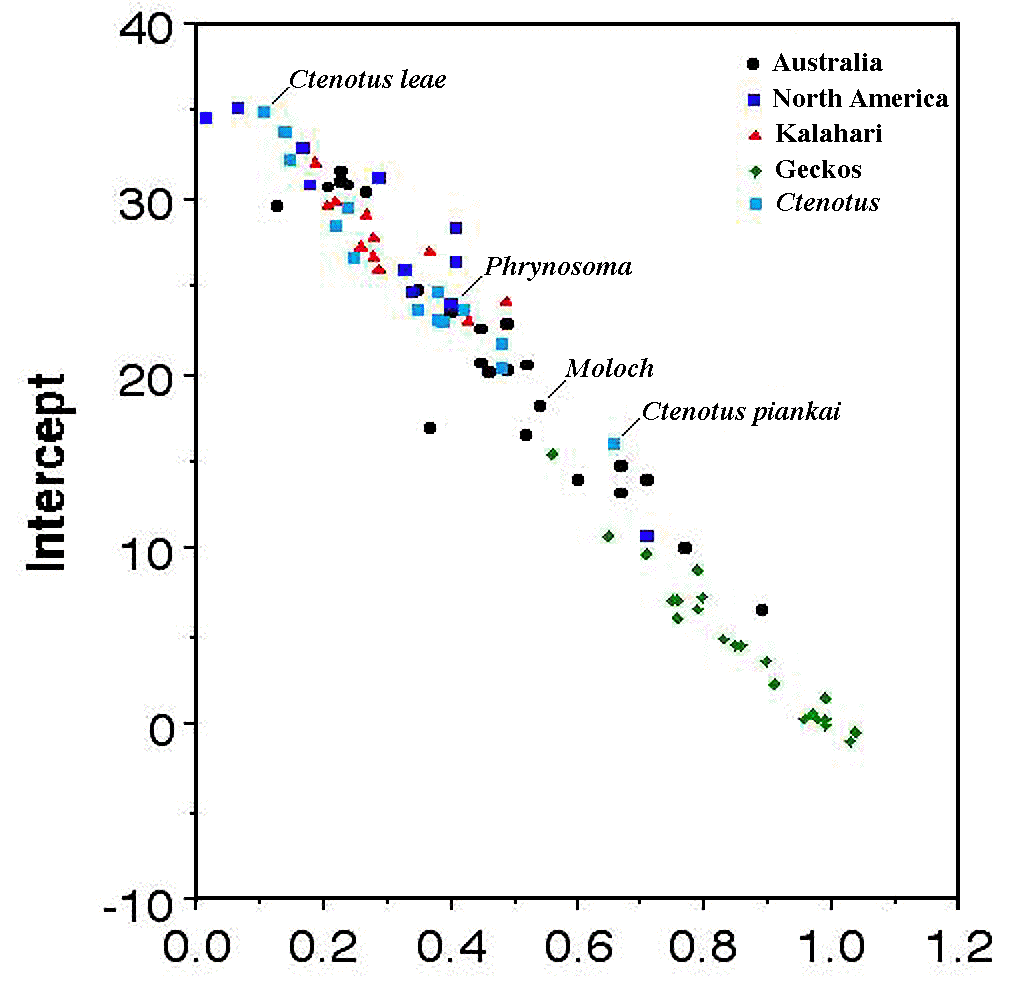 Figure 5.22. Each data point represents the least-squares linear regression of body temperature against air temperature for a given species of desert lizard (data given in Pianka 1986a). Sample sizes are usually substantial (average is 145). The horizontal axis represents the spectrum of thermoregulatory tactics ranging from active thermoregulators (slope of zero) to entirely passive thermoconformity (slope of one).
Squares represent Australian skinks in the genus Ctenotus, which span much of the range of thermoregulatory tactics.
The intriguing "intercept" of the intercepts (38.8°C) approximates the point of intersection of all 82 regression lines and presumably represents an innate design constraint imposed by lizard physiology and metabolism.
Figure 5.22. Each data point represents the least-squares linear regression of body temperature against air temperature for a given species of desert lizard (data given in Pianka 1986a). Sample sizes are usually substantial (average is 145). The horizontal axis represents the spectrum of thermoregulatory tactics ranging from active thermoregulators (slope of zero) to entirely passive thermoconformity (slope of one).
Squares represent Australian skinks in the genus Ctenotus, which span much of the range of thermoregulatory tactics.
The intriguing "intercept" of the intercepts (38.8°C) approximates the point of intersection of all 82 regression lines and presumably represents an innate design constraint imposed by lizard physiology and metabolism.
The intriguing "intercept" of the intercepts (38.8°C) approximates the point of intersection of all 82 regression lines and presumably represents an innate design
constraint imposed by lizard physiology and metabolism. It is presumably not an accident that this value also corresponds more or less to the body temperature of homeotherms, particularly mammals!
Birds, which maintain slightly higher body temperatures than mammals (Hamilton 1973), descended from another reptilian stock, the archosaurs, represented today by the crocodilians. Would a comparable study of crocodilian thermoregulation yield a higher intercept of the intercepts? (This prediction could be doomed to failure by the mere fact that crocodilians are aquatic and very large -- yet they obviously thermoregulate when out of the water.) Although most insects are so small that convective heat exchange prevents them from attaining body temperatures much higher than that of ambient air, some, such as bumblebees and butterflies, do exhibit behavioral thermoregulation; would a plot for insects show more scatter and a different intercept?
Selected References
Limiting Factors and Tolerance Curves
Ehrlich and Birch (1967); Errington (1956); Hairston, Smith, and Slobodkin (1960); Lack (1954, 1966); Liebig (1840); Murdoch (1966a); Odum (1959, 1963, 1971); Shelford (1913b); Terborgh (1971); Walter (1939).
Resource Budgets and the Principle of Allocation
Fitzpatrick (1973); Levins (1968); Randolph, Randolph, and Barlow (1975); Townsend and Calow (1981).
Time, Matter, and Energy Budgets
Emlen (1966); Gadgil and Bossert (1970); Gibb (1956, 1960); Grodzinski and Gorecki (1967); Hickman (1975); Pianka (1976b, 1981); C. Smith (1968); Townsend and Calow (1981); Willson (1972a); Wooten (1979); Zeuthen (1953); Whittaker (1975).
Leaf Tactics
Bailey and Sinnott (1916); Esser (1946a, b); Gentry (1969); Givnish (1979); Givnish and Vermeij (1976); Horn (1971, 1975a, b, 1976); Howland (1962); Janzen (1976); Miller (1979); Mooney et al. (1975); Orians and Solbrig (1977); Parkhurst and Loucks (1971); Ryder (1954); Stowe and Brown (1981); Vogel (1970); Wolf and Hainsworth (1971); Wolf et al. (1972).
Foraging Tactics and Feeding Efficiency
Bell (1991); Charnov (1973, 1976a, b); Charnov et al. (1976); Cody (1968); Emlen (1966, 1968a); Hespenhide (1971); Holling (1964); Huey and Pianka (1981); Kamil and Sargent (1982); MacArthur (1959, 1972); MacArthur and Pianka (1966); Morse (1971); Orians and Pearson (1977); Perry (1999); Perry and Pianka (1997); Pianka (1966b); Pulliam (1974); Rapport (1971); Royama (1970); Schoener (1969a, b, 1971); Schoener and Janzen (1968); Tullock (1970a); Uetz (1992); Werner and Hall (1974); Wolf et al. (1972).
Physiological Ecology
Bligh (1973); Cloudsley-Thompson (1971); Florey (1966); Folk (1974); Gates and Schmerl (1975); Gordon (1972); Guyton and Horrobin (1974); Hadley (1975); Hochachka and Somero (1973); Levitt (1972); Patten and Smith (1975); Prosser (1973); Schmidt-Nielsen (1964, 1975); Townsend and Calow (1981); Vernberg (1975); Vernberg and Vernberg (1974); Wieser (1973); Yousef, Horvath, and Bullard (1972).
Physiological Optima and Tolerance Curves
Brown and Feldmeth (1971); Huey and Stevenson (1979); Ruibal and Philibosian (1970); Schmidt-Nielsen (1975); Shelford (1913b).
Energetics of Metabolism and Movement
Bennett and Nagy (1977); Denny (1980); McNab (1963); Mohr (1940); Nagy (1987); Pearson (1948); Schmidt-Nielsen (1972, 1975); Schoener (1968b); Tucker (1975); Turner, Jennrich, and Weintraub (1969); Zeuthen (1953).
Adaptation and Deterioration of Environment
Fisher (1930, 1958a, b); Henderson (1913); Maynard Smith (1976); Odum (1965); Van Valen (1973).
Heat Budgets and Thermal Ecology
Bartholomew (1972); Bartlett and Gates (1967); Brown and Feldmeth (1971); Brown and Lasiewski (1972); Cowles and Bogert (1944); Dawson (1975); Gates (1962); Hamilton (1973); Huey and Slatkin (1976); Huey and Stevenson (1979); Patten and Smith (1975); Porter and Gates (1969); Porter et al. (1973); Ruibal (1961); Ruibal and Philibosian (1970); Schmidt-Nielsen (1964); Schmidt-Nielsen and Dawson (1964); Wittow (1970); Wieser (1973).
Water Economy in Desert Organisms
Caldwell (1979); Caldwell and Fernandez (1975); Cloudsley-Thompson (1971); Folk (1974); Gindell (1973); Hadley (1975); Main (1976); Schmidt-Nielsen (1964, 1975).
Other Limiting Materials
Feeny (1975); Gilbert (1972); Pauling (1970).
Sensory Capacities and Environmental Cues
Griffin (1958); Lloyd (1965, 1971, 1975); Machin and Lissmann (1960); Schmidt-Nielsen (1975).
Adaptive Suites
Bartholomew (1972); Brown and Lasiewski (1972); Frazzetta (1975); Pianka and Parker (1975b); Rosen (1967); Wilbur (1977).
Design Constraints
Brown and Feldmeth (1971); Duellman and Trueb (1986); Hamilton (1973); Huey and Pianka (1977); Huey and Slatkin (1976); LaBarbera (1983); Liem and Wake (1985); Pianka (1985, 1986a).
|









 Figure 5.9c combines humidity and temperature conditions to show variation in fitness with respect
to both variables simultaneously (a third axis, fitness, is implicit in this figure). The range of thermal
conditions tolerated is narrower at very low and very high humidities than it is at intermediate and more
optimal humidities. Similarly, an organism's tolerance range for relative humidity is narrower at extreme
temperatures than it is at more optimal ones. The organism's thermal optimum depends on humidity conditions
(and vice versa). Fitness reaches its maximum at intermediate temperatures and humidities. Hence, temperature
tolerance and tolerance of relative humidities interact in this example. The concept of a single fixed optimum
is in some ways an artifact of considering only one environmental dimension at a time.
Figure 5.9c combines humidity and temperature conditions to show variation in fitness with respect
to both variables simultaneously (a third axis, fitness, is implicit in this figure). The range of thermal
conditions tolerated is narrower at very low and very high humidities than it is at intermediate and more
optimal humidities. Similarly, an organism's tolerance range for relative humidity is narrower at extreme
temperatures than it is at more optimal ones. The organism's thermal optimum depends on humidity conditions
(and vice versa). Fitness reaches its maximum at intermediate temperatures and humidities. Hence, temperature
tolerance and tolerance of relative humidities interact in this example. The concept of a single fixed optimum
is in some ways an artifact of considering only one environmental dimension at a time.










