7| Evolution and Natural Selection
![]()
Agents of Evolution
Evolution occurs whenever gene frequencies in a population change in time. Individuals do not evolve, but populations do. In addition to natural selection, there are several other agents of evolution, including genetic drift, gene flow, meiotic drive, and mutation. Genetic drift operates by random sampling bias and is confined to relatively small populations. Gene flow occurs by migration movements of plants and animals among and between populations with different gene frequencies. Meiotic drive, or segregation distortion, was briefly considered in Chapter 6. Forward and backward rates of mutation are seldom the same, and the resulting mutation pressure can cause gene frequencies to change. Of these five different agents of evolution, only natural selection is directed in that it results in conformity between organisms and their environments.
Types of Natural Selection
Under stable conditions, intermediates in a population typically leave more descendants, on the average, than do the extremes. We say that they are more "fit." An individual's "fitness" is measured by the proportion of its genes left in the population gene pool. Selection of this sort, which continually crops the extremes and tends to hold constant the intermediate or average phenotype, is termed stabilizing selection (Figure 7.1a). In a stable environment, genetic recombination increases the population's variance each generation, whereas stabilizing selection reduces it to approximately what it was in the previous generation. However, in a changing environment, average individuals (modal phenotypes) may not be the most fit members of the population. Under such a situation, directional selection occurs and the population mean shifts toward a new phenotype (Figure 7.1b) that is better adapted to the altered environment. Eventually, of course, unless the environment continues to change, an equilibrium is reached in which the population is readjusted to the new environment, whereupon stabilizing selection resumes.
A third type of selection, disruptive selection, takes place when two or more phenotypes with high fitnesses are separated by intermediate phenotypes of lower fitness (Figure 7.1c). This usually occurs in distinctly heterogeneous environments with a discrete number of different "patches." Disruptive selection is one mechanism that produces and maintains polymorphisms, such as the green-brown color polymorphisms of many insects. For instance, some butterflies (commonly called "leaf butterflies") mimic leaves; one population may contain both green and brown animals, with the former matching living leaves and the latter dead ones. Through appropriate behavior and selection of matching resting sites, each color morph enjoys a relatively high fitness; in contrast, a butterfly intermediate between green and brown would presumably match its surroundings less well and thus have a considerably lower fitness.
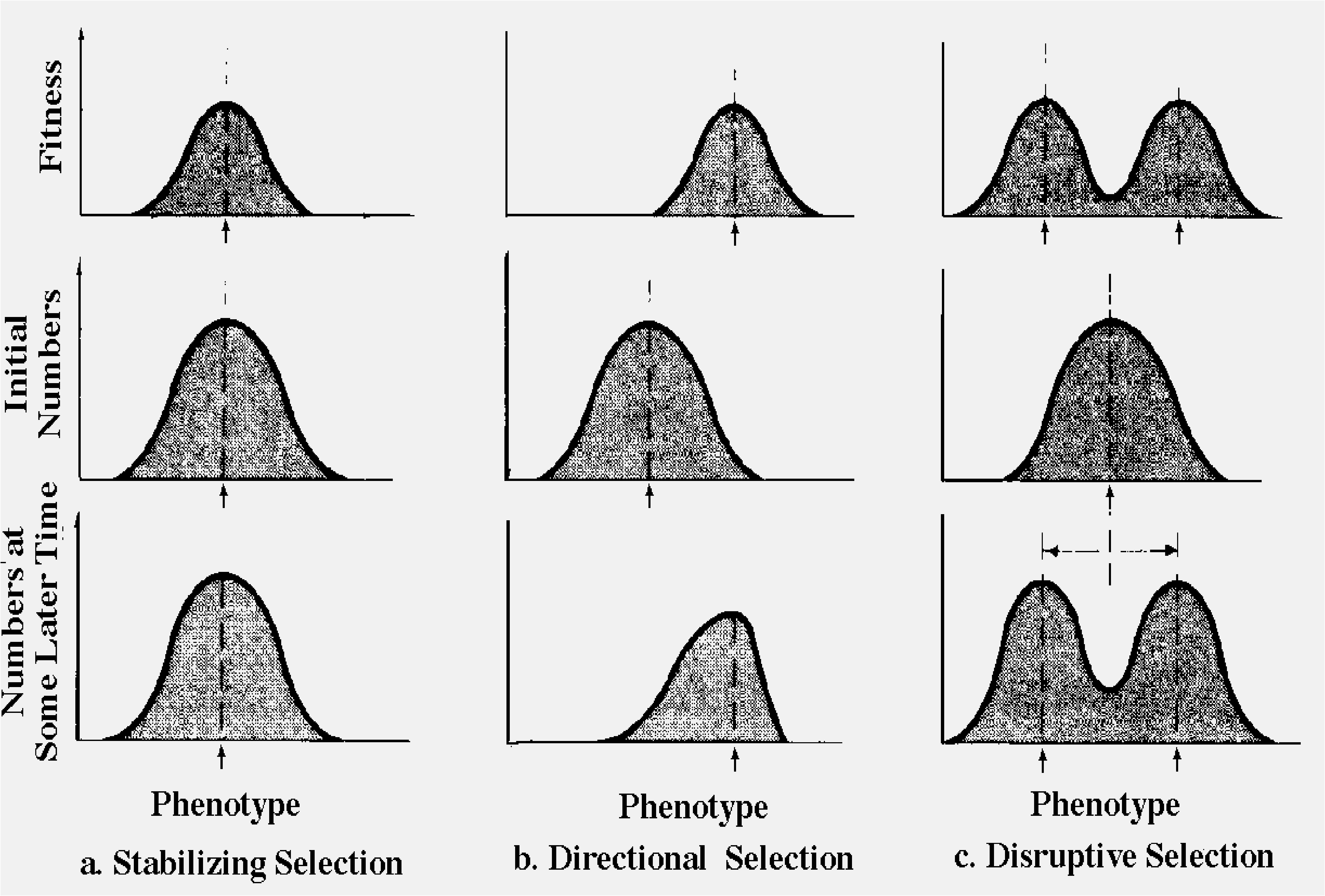 Figure 7.1. Graphic portrayal of the three types of selection. (a) Stabilizing selection, which occurs in constant environments, holds the modal phenotype constant. (b) Directional selection takes place in a changed environment and causes a shift in the modal phenotype. (c) Disruptive selection, with two or more modal phenotypes, occurs in patchy environments with more than one discrete phase.
Figure 7.1. Graphic portrayal of the three types of selection. (a) Stabilizing selection, which occurs in constant environments, holds the modal phenotype constant. (b) Directional selection takes place in a changed environment and causes a shift in the modal phenotype. (c) Disruptive selection, with two or more modal phenotypes, occurs in patchy environments with more than one discrete phase.
Another important type of selection is known as frequency-dependent selection -- this occurs when the fitness of a particular phenotypic trait varies with its frequency in the population. Negative frequency-dependent selection promotes genetic variability. An example would be a bird forming a search image for an abundant prey type, but switching to an alternate prey type when the abundance of the first type is reduced. Such predator switching behaviors favor the rarer prey type which can then increase in abundance until a flip-flop occurs and the predator resumes eating the first prey type again.
Both disruptive selection and frequency-dependent selection can act to help maintain genetic polymorphisms. Other types of selection to be considered in subsequent chapters include age-specific selection, density-dependent selection, density-independent selection, kin selection, and sexual selection.
Ecological Genetics
The European land snail, Cepaea nemoralis, is polymorphic for shell color, with three phases: brown, pink, and yellow. This polymorphism is genetic, based on a three-allele system, with each of the six diploid genotypes varying in color between the endpoints of brown and yellow. In England, a major predator of these snails is the song thrush Turdus ericetorum. These birds break open snail shells on stones ("thrush anvils"). Proportions of shells accumulated at these anvils usually differ from those in the population at large, thereby reflecting the relative intensity of thrush predation on the various snail morphs (Figure 7.2).
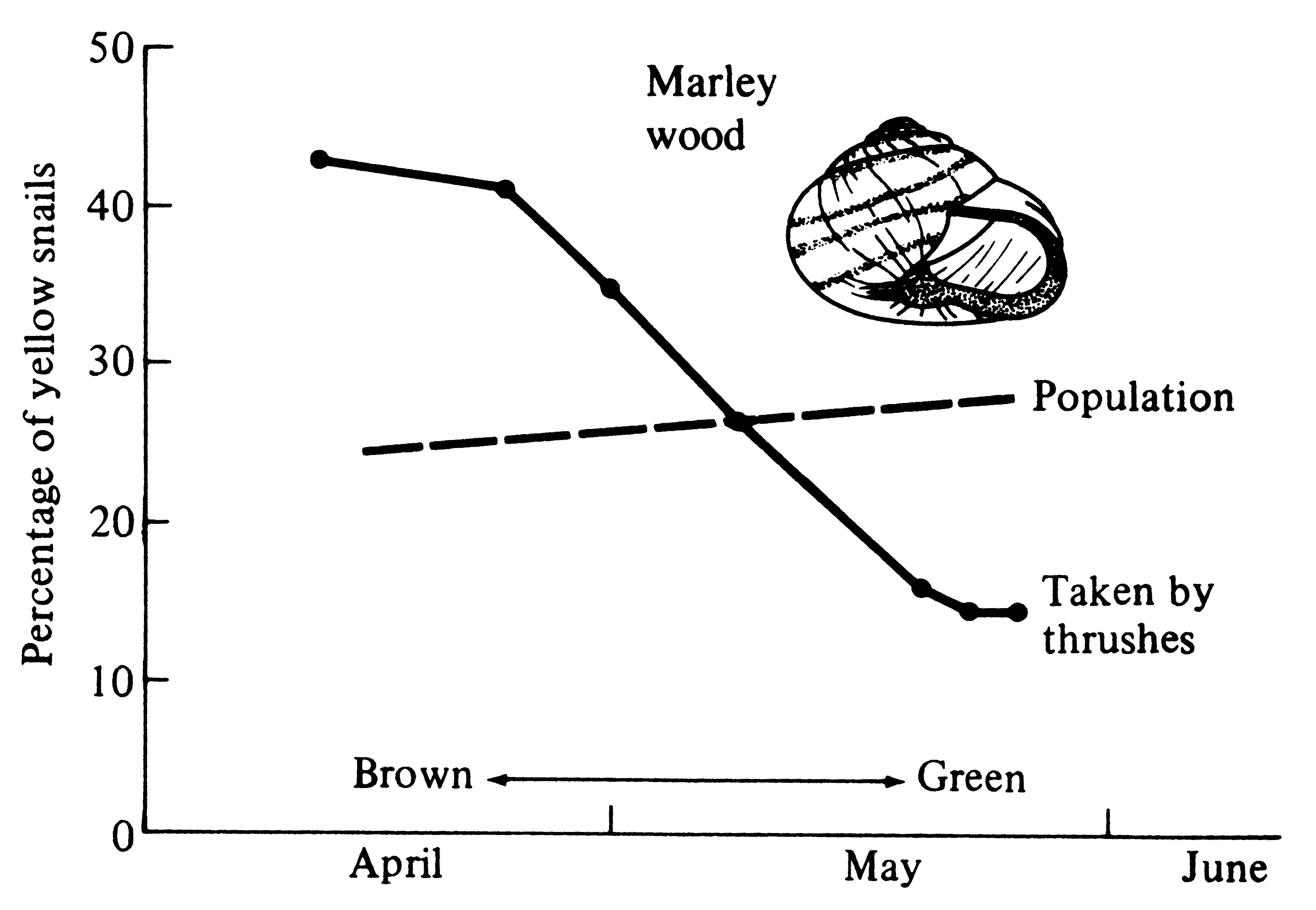 Figure 7.2. Percentages of yellow snails taken by thrushes versus those in the population at large, showing differential predation in time. [From Shepard (1951).]
Figure 7.2. Percentages of yellow snails taken by thrushes versus those in the population at large, showing differential predation in time. [From Shepard (1951).]
During April, available backgrounds in the woods are largely brown; conditions become progressively greener during May. The percentage of yellow shells (with the animal inside, yellow shells take on a greenish hue) found at thrush anvils is higher than in the population at large in April, but lower than in the population in late May (Figure 7.2), indicating differential predation by thrushes. Thrushes form search images for common Cepaea morphs, resulting in negative frequency-dependent selection (Harvey et al. 1975) favoring rare morphs. Thrush predation may well help to maintain the polymorphism in shell color since the brown and pink morphs have a fitness advantage early in the season while yellow snails are at an advantage later.
Allopatric and Sympatric Speciation
How do new species arise? How can one set of interbreeding populations break into two? There are two distinct modes of speciation, the process by which new species arise. Allopatric speciation or geographic speciation occurs in different areas slowly over thousands of years. When the geographic range of a species is broken into two, sets of populations are isolated and gene exchange is prevented, thus allowing such populations to diverge if they are subjected to different selection pressures (this may usually occur when different local conditions require divergent adaptations).
Barriers that can cause such events to occur include glaciers, mountain ranges, oceanic straits and isthmuses, as well as habitat changes caused by long-term shifts in climate. For example, during the pluvial glacial periods in the recent past (about 10,000 years ago), Australia was considerably wetter and an uninterrupted belt of lush mesic forested habitat extended from east to west across the southern third of the continent. Species of birds, frogs, lizards, and other animals had geographic ranges coincident with this contiguous belt of habitat. With the retreat of glaciers and the advent of interglacial conditions (also called interpluvials), an extensive arid area developed in the center of the continent and spread to the south coast. Wetter habitats survive today in the southeast and southwest, but these areas are now separated by a vast arid zone that includes the Nullarbor plain. Closely related species pairs of birds, frogs, and lizards now occur in these southeastern and southwestern refugia.
Due to the lack of gene flow, geographically isolated populations of a species are free to adapt to local conditions. Following an extensive period of experiencing different selective pressures in isolation, the two subsets of what was formerly a single species may have diverged greatly from one another. Whether or not speciation has actually occurred may be put to the test at some later point in time if the two subsets come back together again in sympatry (this might happen, for example, with the advent of another ice age). If the two incipient "species" interbreed and hybridize extensively, the two subsets merge together into one species again in a process known as introgression. However, if the two incipient species are different enough that hybrid individuals suffer reduced fitness, natural selection can favor the evolution of reproductive isolating mechanisms (next section) that prevent introgression from occurring and reinforces the differences between the two new species.
Speciation can also occur more or less instantly, without geographic isolation, and in several different ways. Many species of plants appear to have arisen by the hybridization of two parental species and subsequent chromosome doubling. This process, known as allotetraploidy, occurs as follows: let the two parent species' diploid genomes be represented as AA BB CC and XX YY ZZ, respectively (A, B, C, X, Y, and Z could represent chromosomes). Because the hybrid's genotype is ABCXYZ, chromosomes have no homologues to pair with in meiosis and the hybrid is sterile. However, since the hybrid has attributes of both parental species, it might well be superior to both of them in intermediate habitats (for example, if one parent species is cold-adapted and the other is hot-adapted, the hybrid could outperform both under warm conditions). Such hybrids can survive and even increase in numbers by clonal or vegetative reproduction. Eventually endoduplication or a non-disjunction event during meiosis converts the hybrid's genome from ABCXYZ to AA BB CC XX YY ZZ, restoring fertility (chromosomes can now pair and the hybrid can therefore produce viable gametes). Species that arose via such biparental origin and consequent chromosome doubling are often phenotypically intermediate between their parental species and are often found under intermediate environmental conditions.
Another example of sympatric speciation occurs in tephritid fruit flies (Tephritidae). In most tephritids, males and females are attracted to the same host, which serves as a rendezvous for courtship and mating. Genetic variation in host choice can therefore affect mate choice. Such is the case in Rhagoletis pomonella, which originally oviposited on the fruits of hawthorn. This fly established new host races sypatrically on other members of the plant family Rosaceae, including apples, cherries and roses introduced to North America from Europe. Genetic studies of the apple and Haw populations indicate that they now maintain genetically distinct populations in sympatry (Feder et al. 1988). The sympatric shift from haws to apple involved genetic divergence in genes responsible for host recognition, the timing of adult emergence and differential host related survival (Feder 1998). Adults of the apple and haw populations are now adapted to and mate on different hosts. Thus, the apple population actually represents a newly evolved species (Bush and Smith 1998).
Reproductive Isolating Mechanisms
Many closely related species that do not interbreed in nature have been hybridized in captivity. For example, all pairs of species of falcons (Falco) can produce viable progeny. Mechanisms that prevent interbreeding between species, known as reproductive isolating mechanisms, have frequently evolved.
Isolating mechanisms can be
prezygotic or postzygotic, depending upon whether or not
fertilization actually occurs. Hybrid sterility is an example of
a postzygotic isolating mechanism. Prezygotic behavioral isolating mechanisms can involve
courtship behavior, pheromones, vocalizations, and/or color patterns
that promote species recognition and prevent mismatings from occurring between species. As an
example, female fireflies (actually beetles) respond only to the
flashing pattern and flight paths of males belonging to their own species (Lloyd 1986).
Galápagos Finches
The Galápagos Islands, an archipelago of relatively small and remote, deep water, volcanic islands located about a thousand kilometers west of the Ecuadorian coast (Figure 7.3), support a remarkable group of finches that nicely illustrate a number of evolutionary and ecological principles. These birds dominate the avifauna of the Galápagos. Only 26 species of land birds occurred in the archipelago naturally (i.e., before human introductions), and 13 of these are finches (the islands also support four species of mockingbirds, two flycatchers, two owls, a hawk, a dove, a cuckoo, a warbler, a martin, and a rail).
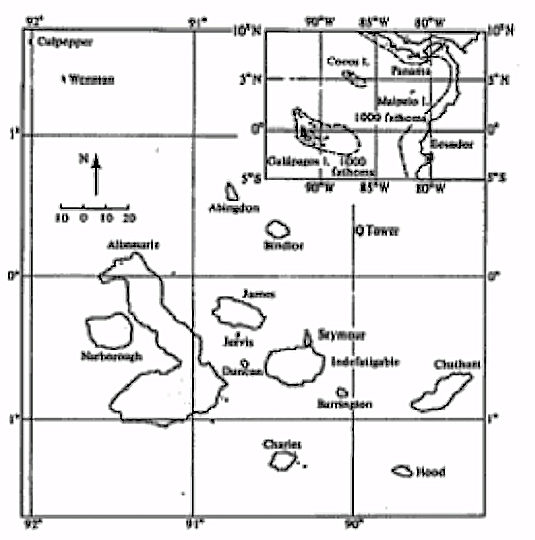 Figure 7.3. Two maps of the main islands in the Galápagos Archipelago. [Inset from Lack (1947). Larger map from Bowman (1961), originally published by the University of California Press. Reprinted by permission of the Regents of the University of California.]
Figure 7.3. Two maps of the main islands in the Galápagos Archipelago. [Inset from Lack (1947). Larger map from Bowman (1961), originally published by the University of California Press. Reprinted by permission of the Regents of the University of California.]
This archipelago (16 major islands and a sprinkling of tiny islets) was formed from volcanic eruptions of the ocean floor about 3 to 5 million years ago; thus originally, there were no organisms on the islands, and their entire biota has been derived from mainland species. Because of their remoteness, relatively few different plant and animal stocks have been able to colonize the Galápagos. (However, the position of these islands on the equator presumably makes them particularly vulnerable to invasions from rafts and floating islands carried out to sea in the equatorial current.)
The 13 species of finches are thought to have evolved from a single mainland finch ancestor that reached the islands long ago. (The birds are similar enough to each other that they are classified as a distinct subfamily of finches, endemic to the Galápagos and Cocos islands.) Archipelagos are ideal for geographic isolation and speciation, especially in land birds such as finches that do not readily fly across wide stretches of water. In such effectively isolated populations, different selective pressures on different islands lead to divergent evolution and adaptations; moreover, occasional interchanges between islands result in heightened interspecific competition, which promotes niche diversification. Drepanid honey creepers underwent a similar adaptive radiation in the Hawaiian islands.
The necessity of geographic isolation and subsequent inter-island colonization for the occurrence of speciation and adaptive radiation is nicely demonstrated by the Cocos Island finch, Pinaroloxias inornata. Cocos Island is a remote and solitary island several hundred kilometers north of the Galápagos and about the same distance from the mainland (Figure 7.3, inset). Cocos Island supports only one species of finch. There has been no opportunity for geographic isolation or reduced gene flow, and the Pinaroloxias gene pool has never split. As might be expected, this finch is a generalist (Smith and Sweatman 1976), probably with a high degree of phenotypic variability between individuals.
Adaptive radiation of these finches in the Galápagos has produced five different genera that differ in where they forage, how they forage, and what they eat (Figure 7.4). So-called ground finches (Geospiza) include six ground-foraging species with broad beaks that eat seeds of different sizes and types as well as flowers of Opuntia cactus. The genus Camarhynchus, termed tree finches because they forage in trees, contains three insectivorous species with generally somewhat narrower beaks; another species (Platyspiza crassirostris) is a vegetarian. Another species, the "woodpecker finch," Cactospiza pallida, uses sticks and cactus spines to probe cracks and crevices for insects, much like a woodpecker uses its long pointed tongue. One very distinctive and monotypic genus, the so-called warbler finch, Certhidea olivacea, is insectivorous and occurs on almost all the islets and islands in the archipelago and breeds throughout most habitats.
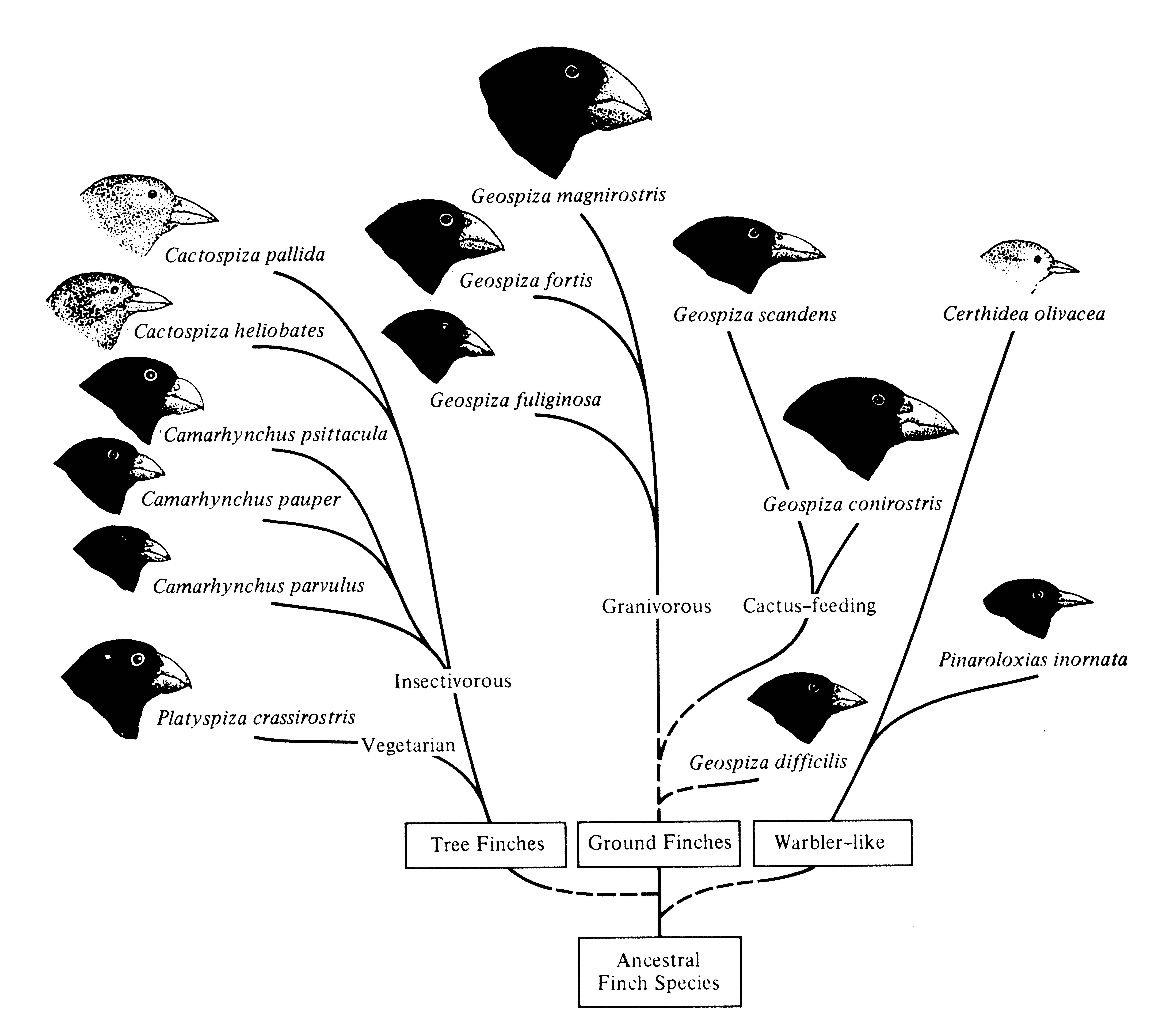 Figure 7.4. Phylogeny of the Galápagos finches. [Phylogenetic tree after Lack (1947); head sketches from Grant (1986) after Swarth and Bowman.]
Figure 7.4. Phylogeny of the Galápagos finches. [Phylogenetic tree after Lack (1947); head sketches from Grant (1986) after Swarth and Bowman.]
From three to ten species of finches occur on any given island in various combinations, although some species have now gone extinct on some islands. Beak lengths and depths are highly variable from island to island (Figure 7.5), presumably reflecting different environmental conditions among islands, including interspecific competitive pressures. Figure 7.5 illustrates character displacement in beak depths; the tiny islets of Daphne and Los Hermanos support only one member of a pair of very similar species, either Geospiza fuliginosa or G. fortis, respectively. On these two small islands, beaks of both species are more similar in beak size than they are on a larger island where the two species occur together in sympatry (Santa Cruz -- upper part of Figure 7.5), where beak depths are completely non-overlapping, with fuliginosa having a small beak (about 6 to 8 mm deep) and fortis a larger beak (about 9 to 15 mm deep). Of course, beak dimensions determine in large part the size of the food items the birds eat, but beaks are also used in species recognition.Beak depths in contemporary finch populations have evolved over the past two decades with changes in seed availability arising from varying climatic conditions:
during drought years, large hard seeds become relatively more available favoring finch individuals with deeper beaks (these have more power and can handle and crush large hard seeds more efficiently).
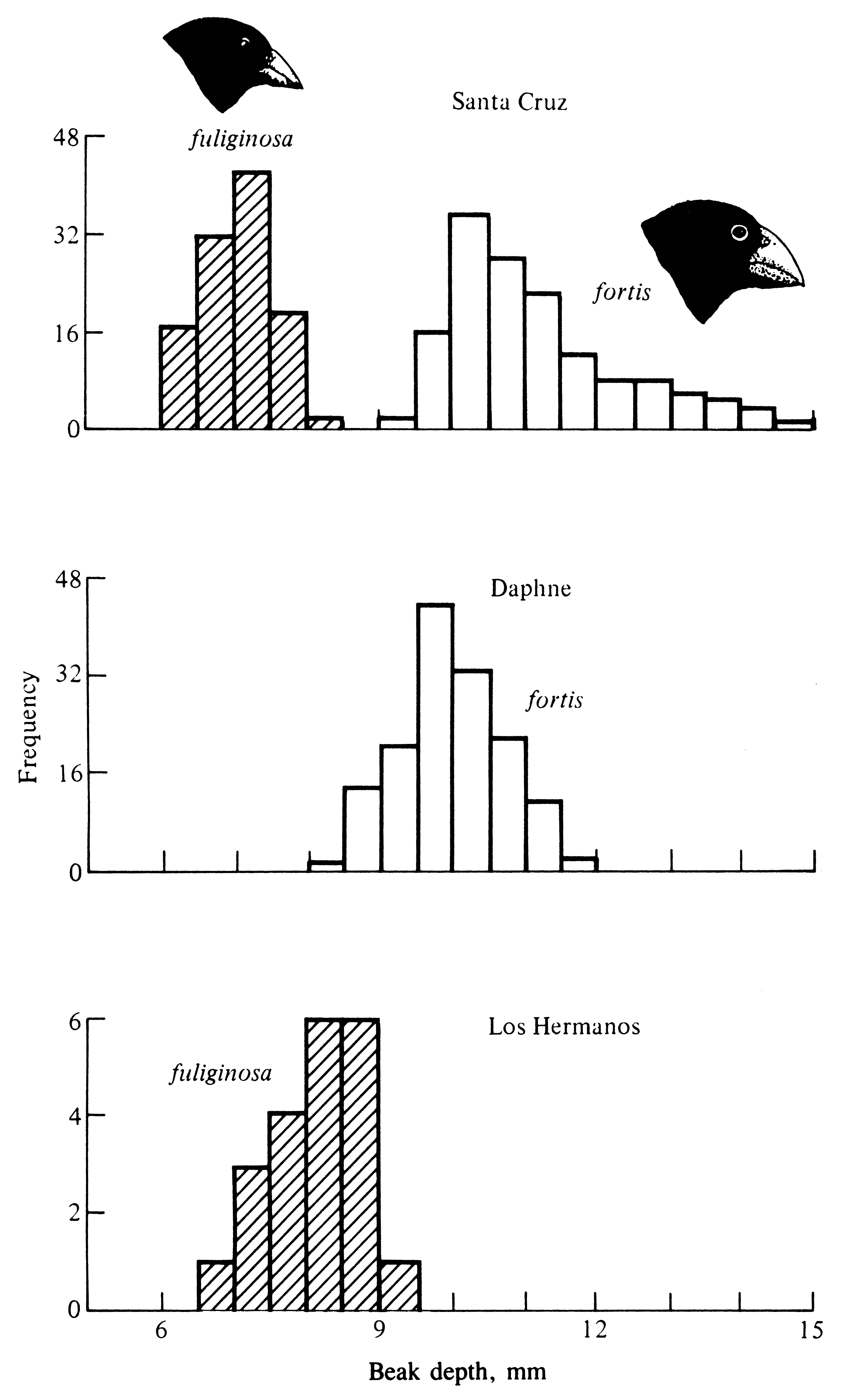 Figure 7.5. Histograms of the beak depths of several species of finches, genus Geospiza, on different islands. In allopatry on the islets of Daphne and Los Hermanos, G. fortis and G. fuliginosa are more similar in beak size than they are in sympatry on Santa Cruz, where their beak depths are entirely non-overlapping. [Adapted from Schluter et al. (1985).]
Figure 7.5. Histograms of the beak depths of several species of finches, genus Geospiza, on different islands. In allopatry on the islets of Daphne and Los Hermanos, G. fortis and G. fuliginosa are more similar in beak size than they are in sympatry on Santa Cruz, where their beak depths are entirely non-overlapping. [Adapted from Schluter et al. (1985).]
Larger islands in the Galápagos Archipelago contain a greater variety of habitat types and, as a result, support more species of finches than do smaller islands. Moreover, the total number of finch species decreases with "average isolation," or the mean distance from other islands, whereas the number of endemic species increases with isolation.
Selected References
Agents of Evolution
Fisher (1930, 1958a, b); Ford (1964); Haldane (1932); Maynard Smith (1958); Wilson and Bossert (1971); Wright (1931).
Types of Natural Selection
Kettlewell (1956, 1958); Mettler and Gregg (1969); Wilson and Bossert (1971).
Ecological Genetics
Cockburn (1991); Dobzhansky (1970); Ford (1964); Harvey et al. (1975); Kettlewell (1956, 1958).
Allopatric and Sympatric Speciation
Bush (1975, 1976); Bush and Smith (1998); Feder et al. (1988, 1998); Futuyma (1987); Mayr (1963); Otte and Endler (1989); Patterson (1982); Prokopy et al. (1982).
Galápagos Finches
Abbott et al. (1977); Bowman (1961); Cox (1995); Grant (1981, 1986); Hamilton and Rubinoff (1963, 1967); Lack (1947); Petren et al. (1999); Schluter (1988); Schluter et al. (1985); Weiner (1994).
|