 The vast majority of genera contain only a few species. Moreover, most species lineages tend to exhibit niche conservatism, seldom changing much in evolutionary time (Holt 1996). Species-rich genera are both rare and extremely interesting because they imply that recent bouts of speciation and niche diversification have occurred, leading to adaptive radiation. Such events of rapid evolution promote species diversity. Major adaptive radiations are uncommon and few have been studied: Hawaiian Drosophila (Carson 1973, 1983, Carson and Kaneshiro 1976, Carson and Templeton 1984), African Rift Lake cichlid fishes (Fryer and Iles 1972, Greenwood 1981, 1984), Galapagos finches (Bowman 1961, Grant 1986, Lack 1947), and Anolis lizards (Losos 1990a 1990b, 1992, Roughgarden 1995, Schoener 1969, 1970, Williams 1969, 1983). The two major lineages of lizards, iguania and scleroglossa, exhibit many fundamental anatomical, behavioral, and ecological differences. For example, iguania are all sit-and-wait foragers whereas scleroglossa are active widely foraging lizards (Cooper 1994). The former group includes Anolis and Sceloporus, the latter includes Ctenotus and Varanus. Iguania have received much more attention than scleroglossa. Ctenotus is the most species rich of all scleroglossan genera. The main goal of the proposed research is to understand factors that facilitated niche diversification and adaptive radiation in this skink genus. The Australian skincid genus Ctenotus has undergone a very extensive adaptive radiation within the last 4-10 million years. It is the most speciose lizard genus (about 100 species) in Australia and among the top five species rich lizard genera in the entire world (others are Anolis, Lerista, Liolaemus, and Sceloporus). The phylogeny of Ctenotus will be recovered by sequencing DNA from mitochondrial and nuclear genes that are evolving at varying rates (to read about our mini-DNA lab, click here). Frozen tissues of 78 species plus 8 subspecies are available. A phylogeny of about 60 species of Ctenotus will be recovered which will allow us to test the monophyly of the genus and current phenetic groupings within Ctenotus. Species groups will be redefined and, in all probability, new species will be discovered.  Comparative Anatomy and Ecology Ctenotus are wary, active, diurnal, heliothermic lizards. Members of this genus have radiated into a wide variety of habitats, including tropical and temperate forests, savannas, mulga scrub, shrubby habitats, dry lakebeds, mallee, rocky habitats, coastal sands, desert sandridges, and desert sandplains. Except for Tasmania and on high mountain tops, Ctenotus occur virtually anywhere one goes in Australia. 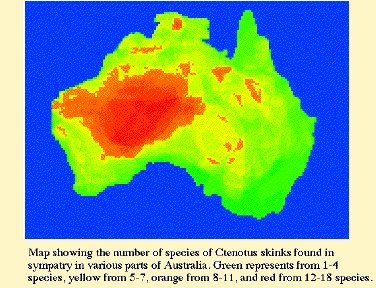

In an ecological study of 14 species belonging to 7 different species groups, Pianka (1969, 1986) found up to 11 species in sympatry with 7 sympatric species being usual in the Great Victoria desert. James (1991a, 1991b) found 5 species sympatric in deserts of the central ranges. Distinct differences in microhabitat and habitat requirements occur among members within three putative species groups. In the atlas group, for example, piankai and quatt. spend most of their time within spinifex tussocks on sandplains, whereas dux is associated with sandridge habitats. Members of the colletti group have also evolved differences in habitat specificity: Ctenotus calurus lives on sandplains, whereas the putatively closely related leae and colletti are sandridge species. Ctenotus schomburgkii are found on in shrub-Acacia habitats and on sandplains, whereas its close relative brooksi is restricted to sandridges. These observations suggest a high level of lability in habitat specificity û however, this interpretation depends upon recognition of monophyletic species groups. Phylogenetically informative data are greatly needed to test current arrangements of species into putative groups within Ctenotus. Given a phylogenetic hypothesis for Ctenotus, the following sorts of hypotheses about character evolution can be tested. H 1: Evolution of diet: Dietary generalization is primitive and specialization is derived. Some species of Ctenotus (ariadnae, pantherinus, schomburgkii) are extreme food specialists eating only termites, whereas other species (brooksi, dux, piankai, quatt.) have quite broad diets (Pianka 1986). Ancestral states for dietary niche breadth will be estimated (Huey and Bennett 1987; Maddison 1991; Martins and Garland 1991; Maddison and Maddison 1992; Martins and Hansen 1997). The hypothesis that dietary generalism is primitive will be tested. Whether highly specialized lineages within Ctenotus have more species will also be ascertained. H 2: Evolution of toe scales (lamellae): Wide calli are ancestral, fine sharp keels are derived. Luke (1986) documented multiple independent origins of fringed toe lamellae among many different lizard families, suggesting that lamellar morphologies might be of adaptive importance, while Arnold (1983, 1989) suggested that toe lamellar morphologies may be adaptations to specific substrate types and ambient thermal conditions. Several different distinct types of subdigital toe lamellae occur among Ctenotus. Outgroup analysis suggests that wide calli are the ancestral condition within Ctenotus. Preliminary analysis suggests that several reversions to broad calli have occurred. Fine sharp keels appear to have arisen within the group at least twice. 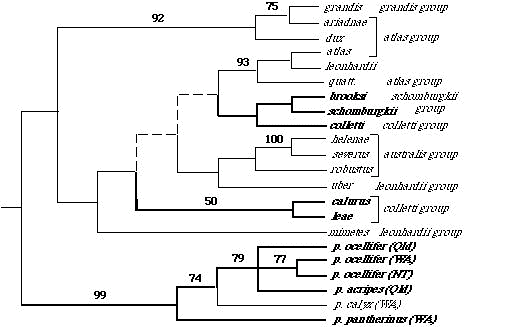 Fig. 1. Ctenotus phylogeny inferred from 12S and 16S rDNA data (826 aligned positions 118 informative characters). This strict consensus tree is based on 4 equally parsimonious trees (CI=0.667). Numbers above branches denote bootstrap proportions from 200 bootstrap replicates. Scincella lateralis, Hemiergis decresiensis and Eremiascincus richardsoni were simultaneously used as outgroup taxa. Abbreviations next to the C. pantherinus subspecies designate Australian states where individuals were collected (Qld=Queensland, NT=Northern Territory, WA=Western Australia). Light italics represent taxa with calli, boldfaced taxa are those with sharp keels, heavy lines depict probable lineages with sharp keels whereas light lines depict the broad callous condition. Dashed lines indicate ambiguity. Of special interest to the polarity of toe lamellar morphologies is the phylogenetic status of C. pantherinus, which has the largest geographic range of any Ctenotus, and appears to be the sister taxon to other Ctenotus. C. pantherinus is exceptional in displaying different toe lamellar states between recognized subspecies: calyx has broad calli while acripes, ocellifer and pantherinus have sharp keels. Tissues and voucher specimens for all putative subspecies of pantherinus are available. Two disjunct populations of subspecies acripes exist, one occurs in extreme NW WA on Barrow Island, the other occurs in Queensland. Toe lamellae of these two allopatric populations differ markedly, suggesting that they are different taxa. Phylogenetic relationships are needed to confirm and interpret these patterns of evolution. Subspecies of pantherinus appear to form a monophyletic group in which sharp keeled toe lamellae have evolved independently but reverted back to broad calli in calyx. Toe lamellae are fairly stable within most putative species groups, even in those that include species that live on different soil surfaces and encounter different ground temperatures. Furthermore, species with the full range of toe lamellae occur together in sympatry in the desert, calling into question the importance of substrate type as the only factor in the evolution of toe lamellae. My graduate student A. Gluesenkamp has begun a detailed scanning electron microscopic study of subdigital lamellae of Ctenotus species, and he will attempt to relate toe lamellar condition to substrate textures and temperatures. H 3: Limb evolution: Open foraging is associated with longer hindlegs, while use of porcupine grass tussocks is associated with shorter hindlegs. Species that forage in the open have relatively longer hindleg lengths (expressed as a percentage of snout-vent length) than do species that stay in spinifex grass tussocks. If such a relationship still holds after effects of relatedness have been removed by tests of independent contrasts, this will suggest that leg length evolves along with microhabitat utilization. Body size would appear to have evolved rapidly within groups as well: leae is larger than calurus or colletti, and grandis is considerably larger than its putative sister species hanloni (for a phylogenetic analysis of the evolution of body size see Pianka 1995). Huey and Bennett (1987) and Garland et al. (1991) examined the influence of phylogeny on coadaptation of thermal physiology in some Australian skinks, including several species of Ctenotus. Their work, plus Greer's (1980) comments, suggest that most, but not all, Ctenotus display appreciably higher body temperatures than related skinks. Apparently, acquisition of this "key innovation" (Simpson 1953, Futuyma 1986; Nitecki 1990) allowed Ctenotus to become active diurnal lizards and to radiate to fill many new niches. An alternative hypothesis is that aridification itself has allowed these lizards to diversify (see H 6). Huey and Slatkin (1976) analyzed costs and benefits of lizard thermoregulatory tactics, and identified the slope of the regression of body temperature against ambient environmental temperature as a useful inverse measure of the degree of passivity in regulation of body temperature. On such a plot of active body temperature versus ambient temperature, a slope of one indicates true poikilothermy or totally passive thermoconformity (air temperature and body temperature are perfectly correlated), whereas a slope of zero reflects the other extreme of perfect thermoregulation. Lizards span this entire thermoregulation spectrum (Pianka 1986). Among active diurnal heliothermic species, regressions of body temperature on air temperature are fairly flat (for several species, including some quite small ones, slopes do not differ significantly from zero). Both thermoregulators and some thermoconformers maintain higher body temperatures than do species intermediate between them along this thermoregulatory spectrum. These thermoconformers, Ctenotus ariadnae, quatt. and piankai, maintain relatively high body temperatures by being active during the heat of mid-day inside spinifex tussocks. At least two evolutionary pathways for increasing body temperature are suggested. The hypothesis that elevated body temperatures is derived can be tested by comparative methods, as well as the hypothesis that each thermoregulatory strategy has evolved only once within Ctenotus. A comprehensive study of evolution of thermoregulation within Ctenotus will be undertaken using modern comparative methods (Huey and Bennett 1987, Garland et al. 1991). Body temperatures at ancestral nodes will be estimated. The notion that elevation of body temperature has been important in the adaptive radiation of Ctenotus suggests two testable hypotheses: H 4: Higher body temperature is a derived condition within Ctenotus, and has arisen multiple times. H 5: Lineages that have evolved higher body temperatures are more species rich than those which are not. This comparative phylogenetic study will lead to a much more sophisticated understanding of Ctenotus ecology. As described above, some Ctenotus species, such as leae and leonhardii, are active thermoregulators whereas other species, such as piankai, are, comparatively speaking, thermoconformers. The position of any particular species along this thermoregulation spectrum reflects a great deal about its complex activities in space and time. Thermoregulatory behavior provides a convenient unidimensional surrogate for multi-dimensional spatial-temporal niche dimensions (Pianka 1993), and offers a potent linear dimension on which various species can be placed in attempts to formulate general schemes of lizard ecology (Pianka 1985, 1986). Various other ecological parameters, such as reproductive tactics, can also be mapped on to this emergent spatial-temporal axis (Pianka 1993). Correlations between thermoregulatory and ecological/life history characters can be tested for statistical significance after controlling for phylogenetic relationship using modern comparative methods. How has this remarkably species rich genus undergone such extensive speciation so rapidly within continental Australia? Relatively few obvious geographic barriers, such as mountains, lakes, and/or rivers, exist in the Australian desert region. Zones of unsuitable habitat form the most effective barriers to lizard dispersal. The high degree of habitat specificity observed in Australian desert lizards, coupled with extensive spatial patchiness in the mosaic of desert vegetation, led me long ago to propose a simple model for lizard speciation based on fluctuating boundaries among three major habitat types to which Australian desert lizards have evolved habitat specificity (Pianka 1969a, 1972). Some species of Australian desert lizards are restricted to flat spinifex sandplains, others to shrub-Acacia (mulga) habitats, and still others to sandridges. Habitat-specific lizard species, such as those restricted to shrub-Acacia ("mulga") habitats, move along appropriate corridors. Long-term changes in climate and/or soils, such as movements of windblown sands, could easily break shrub corridors, separating eastern from western patches of shrub-Acacia habitats and isolating shrub-specific lizards to diverge and speciate. Simultaneously, sand corridors are opened, allowing sand-specialized stocks to invade previously unoccupied sandy desert regions. Later in geological time, shifting sands could reverse the process, resulting in closure of sand corridors, forming isolates among sand species while reopening shrub corridors. Such alternating habitat junctures probably isolated eastern from western populations of lizards restricted to shrub-Acacia habitats as well as northern from southern (in central and western Australia) and eastern from western populations of species restricted to sandridge and/or sandplain-Triodia habitats (see maps in Pianka 1972). In support of the above hypothesis, the sandridge-specialized Ctenotus brooksi species complex has several recognized taxa, euclae in the coastal sand dunes along the south coast, iridis in the Victorian Little desert, taeniatus on the sands surrounding Lake Torrens, aranda in the Simpson desert, and brooksi in the Great Victoria and Gibson deserts. Knowledge of phyletic relationships of these taxa will provide exceedingly valuable insights into vicariant events that took place during the history of arid Australia. A number of putatively closely-related species pairs also live together in the same habitat but have evolved other niche differences, such as colletti and leae, as well as piankai and quatt. Do closely-related sympatric species diverge in ecology more than distantly-related species? With a phylogeny, one can test whether or not various assemblages actually known to coexist in various geographic regions or at particular study sites are either more or less closely-related than expected by comparing their phylogenetic distances against sets of species drawn at random in repeated Monte-Carlo simulations (Pimm 1983, Gotelli and Graves 1996). Ecological similarities will also be compared with phylogenetic distances (Haydon et al. 1993). As mentioned above, members of some Ctenotus species groups, such as the putative colletti and schomburgkii groups, contain species that have become specialized to different habitat elements, suggesting that habitat specificity has evolved (brooksi, colletti and leae are sandridge species, whereas putatively closely-related calurus and schomburgkii are denizens of sandplain-Triodia habitats. In the putative atlas species group, dux is a sandridge species, whereas piankai and quatt. are sandplain species. It will be most instructive to overlay habitat preferences on a resolved phylogeny to determine the extent of evolutionary lability in habitat specificity. In support of Lack's (1968) hypothesis, Richman and Price (1992) found that habitat difference evolved last in Palearctic leaf warblers. Will the same conclusion hold true for these desert lizards? Historical Biogeography and Evolution of Arid Zone Squamates The time frame for the evolution of the genus Ctenotus is closely comparable to current estimates of the period of aridification of the Australian continent. H 6: Ctenotus have radiated in response to the progressive enlargement and diversification of the arid zone. If so, then a biogeographic analysis of Ctenotus, undertaken within a phylogenetic framework, should reveal a wealth of information about the process and history of aridification itself. Pertinent questions include: 1) Are arid-adapted Ctenotus derived from within species groups of semiarid and temperate origin, or is the reverse true?* 2) Have various habitat "islands" of "hard" country played a role in the evolution of Ctenotus? Six other Australian squamate genera are also species-rich: [Lerista (59 spp.), Diplodactylus (36 spp.), Egernia (28 spp.), Varanus (25 spp.), Ctenophorus (21 spp.), Ramphotyphlops (31 spp.)]. These need to be examined in a comparative sense. Ctenotus, the most species rich and most widespread genus, represents the ideal test case for development of methods and will provide an incentive for equivalent studies in other groups. Obviously, congruence will lend credence to hypothesized vicariant events. Overall vertebrate/reptile area cladograms for Australia were developed by Cracraft (1991), who found multiple equally parsimonious area cladograms which could be tested by addition of detailed information on Ctenotus. Because a number of species of Ctenotus are endemic to Cracraft's extensive "interstitial" regions, a more fine-scale analysis will be undertaken and an area cladogram constructed for the genus Ctenotus, which will be compared with Cracraft's faunal cladograms. Finally, such studies will also supply the context for predictions regarding diversity of other groups of arid zone animals/plants and will have obvious management implications, especially with regard to conservation of genetic diversity. All sequence data will be archived in GenBank, making these data available to everyone. This study will elucidate factors allowing a major adaptive radiation and will produce a phylogeny that will be used. I anticipate first publishing a series of papers and then editing a synthetic multi-authored volume with a University press. This will include papers and chapters on both (1) molecular and (2) morphologically based phylogenies, plus (3) a combined total evidence analysis, (4) a taxonomic rearrangement of species groups, (5) descriptions of new species (6) analyses of biogeography and geographic distributions, (7) evolution of microhabitat utilization and thermoregulation, (8) evolution and function of toe lamellae, (9) evolution of body size, (10) evolution of habitat preference, (11) evolution of diet, (12) evolution of reproductive tactics, and (13) an overview of key innovations and environmental opportunities that allowed this adaptive radiation. Biogeographic and phylogenetic studies will always be plagued by the fact that the past is singular, unknown, and we cannot return to it. Soft body parts, behavior and physiology do not fossilize. With information of the sort that will be gathered here, however, much can be inferred about past history û- probable regions of origin, speciation events, and dispersal events can be identified. Probable ancestral states will be inferred from those of surviving descendants. Once anatomical and ecological convergences have been elucidated via phylogenetic systematics and appropriate phylogenetically-based, multivariate statistical analyses, testable predictions can be made. Results of this systematic study will enable prediction of probable ecologies of other, as yet unstudied, species of Ctenotus and other taxa (both plant and animal), which can be tested by direct examination in the field. Community ecology has usually been perceived to be a descriptive science, but phylogenetic systematics will enable it to finally become predictive. This study will expand our understanding of evolution and niche diversification as well as spawn numerous other interesting new lines of inquiry. |