 Great Australian Lizard Expedition
Great Australian Lizard Expedition

I saw my first lizard when I was about 6 years old: It was a gorgeous, green, sleek, long-tailed arboreal creature (an Anolis carolinensis) climbing around in some vines. My uncle and I did our utmost to capture that lizard, but all we were able to get was its tail. I remember stamding there holding its twitching tail, wishing intensely that it was the lizard instead. A year later, I caught my first garter snake, which I tried to keep as a "pet," however, it soon escaped (snakes are escape artists!). In the third grade, I discovered a captive baby alligator in the classroom next door. That alligator transfixed me -- I stood by its aquarium for hours reveling in its every move. As a small boy, I was destined to become a biologist, long before I had any inkling whatever about what science even was. Years later, in graduate school, I discovered the layers in the biological cake, and eventually I went on to earn a Ph.D., and, later, my D. Sc. as a professional ecologist. My boyhood hometown, Yreka, in far northern California, was a sleepy little town of about 3,500 people in those days, surrounded by relatively pristine wilderness. My life as a young boy was rich, replete with the great outdoors and lots of adventure. It was an easy walk out our back door to a variety of relatively undisturbed natural habitats. After school, my brother Mike and I roamed the juniper-covered hills around town catching snakes and lizards. We lived outside and knew virtually every hectare within several miles of town. I collected everything from rocks to insects (especially butterflies) to bird eggs to lizards and snakes, including rattlesnakes. My collections were semiprofessional, too, with every specimen dutifully labeled with its scientific name. I went to great trouble to build a glass display case to house my collection of bird eggs, and we went to even greater lengths to find the nests of all the local breeding species, ranging from hummingbirds to great horned owls. I kept snakes and lizards alive, and when they died, dutifully pickled them in denatured alcohol as proper museum specimens. In graduate school, I became interested in the challenge of understanding species diversity: why are there more species in some places than in others? A prominent geographical pattern, repeated in many different groups of plants and animals, became the focus of my research: latitudinal gradients in species diversity. I chose to study the ecology and diversity of desert lizards in flatland western North America, along a 1,000-km latitudinal transect from southern Idaho through southern Arizona. For 3 field seasons, I lived out of a Volkswagon van, driving up and down this transect collecting data. Five years later, I finished my Ph.D. The number of species of lizards occurring together varied from 4 to 10 and was correlated with the structural complexity and spatial heterogeneity of the vegetation. Northern deserts support a homogeneous vegetation consisting of small chenopodiaceous shrubs, including sagebrush and saltbush (Atriplex). Southern deserts support a much more complex vegetation which includes a variety of small semi-shrubs, larger woody shrubs including creosote bush (Larrea), scattered trees such as Joshua "trees," palo verde, and ironwood, plus various sorts of cactus. Several species of climbing lizards require large shrubs and trees. The Adaptive Suite of Horned LizardsVarious features of anatomy, behavior, diet, temporal pattern of activity, thermoregulation, and reproductive tactics of horned lizards can be profitably interrelated and interpreted to provide an integrated view of the ecology of these interesting lizards. Horned lizards are ant specialists. Some species eat essentially nothing else, while other lizard species eat a wider variety of insects. Ants are small and contain much indigestible chitin, so that large numbers of them must be consumed. As a result, an ant specialist must possess a large stomach for its body size. Consider the desert horned lizard Phrynosoma platyrhinos in greater detail. When expressed as a proportion of total body weight, the stomach of this horned lizard occupies a considerably larger fraction of the lizard's overall body mass (about 13 percent) than do the stomachs of all other sympatric desert lizards, including the herbivorous desert iguana Dipsosaurus dorsalis (herbivores have lower assimilation rates and typically have larger stomachs than carnivores). Possession of such a large stomach requires a tank-like body form, reducing speed and decreasing the lizard's ability to escape from predators by flight. Natural selection has therefore favored a spiny body form and cryptic behavior rather than a sleek body and rapid movement to cover (as in the majority of other species of lizards). Risks of predation are likely to be increased during long periods of exposure while foraging in the open. A reluctance to move, even when actually threatened by a potential predator, could well be advantageous. Movement might attract attention of predators and negate the advantage of concealing contour and coloration. Such decreased movements contribute to the observed high variance in body temperature of Phrynosoma platyrhinos, which is significantly greater than that of all other species of sympatric lizards. 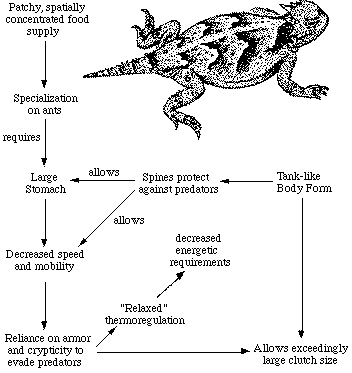
Phrynosoma platyrhinos are also active over a longer time interval than any sympatric lizard species. Wide fluctuations in horned lizard body temperatures under natural conditions presumably reflect both the long activity period and perhaps their reduced movements into or out of the sun and shade (most of these lizards are in the open sun when first sighted). More time is thus made available for activities such as feeding. A foraging anteater must spend considerable time feeding because ants are so small that many must be eaten. Food specialization on ants is economically feasible only because these insects usually occur in a clumped spatial distribution and hence constitute a concentrated food supply. Desert horned lizards trapline from ant mound to mound and eat over 200 ants a day. As explained above, to make use of this patchy and spatially concentrated, but not overly nutritious, food supply, P. platyrhinos has evolved a unique constellation of adaptations that include a large stomach, spiny body form, an expanded period of activity, and relaxed thermoregulation (eurythermy). The high reproductive investment of adult female horned lizards is probably also a simple and direct consequence of their robust body form. Lizards that must be able to move rapidly to escape predators, such as whiptail lizards (Aspidoscelis), would hardly be expected to weight themselves down with eggs to the same extent as animals like horned lizards that rely almost entirely upon spines and camouflage to avoid enemies. While working on my Ph.D. dissertation, I conceived of an ideal follow-up research project: namely, to compare an independently evolved desert-lizard system with the one I had just studied. I had already begun to dream about the possibility of one day emigrating to Australia, and so it was natural to propose as a postdoctoral project the comparison of Australian desert lizards with those in North America. I wondered whether two independent desert-lizard systems isolated by 200 million years of separate histories would converge. I applied for, and was awarded, a 3-year stipend as a National Institutes of Health postdoctoral fellow to work with the world's leading ecologist, the late Professor Robert H. MacArthur, at Princeton University. In November of 1965, I went to Princeton and wrote a companion proposal to the National Science Foundation to support the proposed fieldwork in Australia (this was also funded, for MacArthur agreed to be a co-principal investigator). In December, I returned to the West Coast, and married a graduate school colleague -- now my ex-wife Helen. She was a laboratory biologist but planned to accompany me doing extensive fieldwork. We spent May and June 1966 driving brutal dirt roads half the length of Baja California, then a real wilderness area (before the paved highway was constructed). We censused lizards on low shrubby flat desert areas, comparable to sites I had studied farther north. We found a flatland desert site in Baja, structurally comparable to my study sites in the Great Basin, with five different species of lizards coexisting together. At any given site in the flatland deserts of western North America, the exact number of lizard species present depends primarily on the structural complexity and spatial heterogeneity of the vegetation. I expected to find similar patterns in Australian deserts. I was especially interested in making detailed comparisons of so-called ecological equivalents, independently evolved lineages that occupy roughly the same ecological roles on different continents. A prime example would be North American horned lizards, which are in a different family than their Australian counterpart the thorny devil Moloch horridus. Would Australian thorny Devils match the adaptive suite I had discovered in North American Phrynosoma? Such products of convergent evolution are of great interest to biologists because they suggest that natural selection favors predictable responses to particular environmental exigencies. Moreover, the mere existence of such convergent species pairs indicates that evolutionary pathways can be predictable and repeatable. I had hopes of not only documenting such evolutionary convergence in detail, but also perhaps contributing new ideas about the vital process of natural selection itself. A major focus of my life's work has been to understand species diversity, or species richness, of desert lizards. Understanding why more species occur at some places than at others has been a focal point of my research for many years. In graduate school during the early 1960Ős, my Ph.D. research was on species diversity of North American desert lizards. The first chapter of my dissertation was a review of concepts of latitudinal gradients in diversity, published in the American Naturalist in 1966. It has been reprinted in several collections of important papers and was celebrated at a symposium in 2016 entitled "Latitudinal Gradients in Species Diversity: 50 years since Pianka". After publishing a companion paper on species diversity of lizards in North America, I embarked on a 3-year postdoctoral with Robert H. MacArthur at Princeton University, 18 months of which was spent studying the ecology and diversity of Australian desert lizards. My ex-wife Helen and I found the most diverse lizard assemblages known. The richest site, Redsands, has 55 different species of lizards in sympatry. We discovered half a dozen as yet undescribed species of comb-eared skinks (genus Ctenotus), two of which are named after us. During 1970, with the able assistance of Raymond Huey, I extended these studies to include the Kalahari Desert in southern Africa.  Lizard specimens were also dissected and their sex and reproductive condition assessed. Stomach
contents were analyzed and summarized. Ten body measurements were made on preserved specimens.
Many of these supplementary data remain at risk and will be lost with Pianka's demise unless he
can hire a full-time research assistant to help get them digitized and entered into a data base.
Lizard specimens were also dissected and their sex and reproductive condition assessed. Stomach
contents were analyzed and summarized. Ten body measurements were made on preserved specimens.
Many of these supplementary data remain at risk and will be lost with Pianka's demise unless he
can hire a full-time research assistant to help get them digitized and entered into a data base.
The culmination of Pianka's life and work is now at stake -- as much information as possible for each individual lizard should be preserved for posterity. These data need to be organized in a relational database format describing different types of data with queries to extract particular subsets. These data will constitute an invaluable resource for a wide variety of future studies, including long-term changes, ontogenetic changes, variation and sexual dimorphisms in morphology and ecology, habitat requirements, thermal biology, reproductive biology, within- versus between-phenotype components of niche breadth, as well as dietary and microhabitat niche breadth and overlap. |