|
(under Construction) © Eric R. Pianka Over the past 50 years, I have collected relevant anatomical and ecological data on nearly a hundred species of desert lizards in a dozen different families (clades) on three continents. Sample sizes are adequate for gender comparisons for many species, but a few uncommon species could not be included. Here I present selected data illustrating some gender differences and discuss these from within a phylogenetic context. Sex Ratios and Behavioral Differences Males are especially vulnerable to predation during the breeding season when they must search for females. Gravid females tend to be much more secretive and are usually difficult to find. As a result, apparent sex ratios are often biased towards males, although this could well be an artifact of sex-specific behavioral differences. Species population sex ratios are statistically biased towards males in 26 of the 92 species and significantly biased in favor of females in only 5 species. Sex ratios did not differ significantly from 50:50 among the remaining 61 species. Among 11 North American species, sex ratios do not depart from equality except in the teiid Apidoscelis tigris, where the ratio is significantly skewed in favor of males (56.34% + 2.96). Sex ratios depart significantly from 50:50 in 5 of the 20 Kalahari species with 3 lacertid species (Heliobolus lugubris, Meroles suborbitalis and Pedioplanis lineoocellata) biased towards males and two skink species (Trachylepis (formerly Mabuya) spilogaster and Typhlosaurus lineatus) towards females. Skewed sex ratios are more frequent among 61 Australian species, with significant male dominance in 22 species: 7 species of Ctenotus skinks plus 3 other skink species (Egernia inornata, Lerista desertorum, and Morethia butleri), as well as in two pygopodid species (Delma butleri and Lialis burtionis), 4 gecko species (Diplodactylus conspicillatus, Nephrurus laevissimus, N. levis, and N. vertebralis), and two varanids (Varanus eremius and V. gouldii). Among Australian desert lizards, female bias occurs in two agamids (Moloch and Pogona) and in the gecko Heteronotia some populations of which are parthenogenetic. Morphological Dimorphisms Anatomical trade offs between the sexes are often observed, although their functional significance often remains somewhat elusive. In most species, females tend to be slightly larger than males. The most plausible explanation for this is the fecundity advantage females gain with increased body size. For example, in most lizard species including the thorny devil (Moloch horridus), clutch size increases with body size and females are larger than males (Pianka and Pianka 1970). 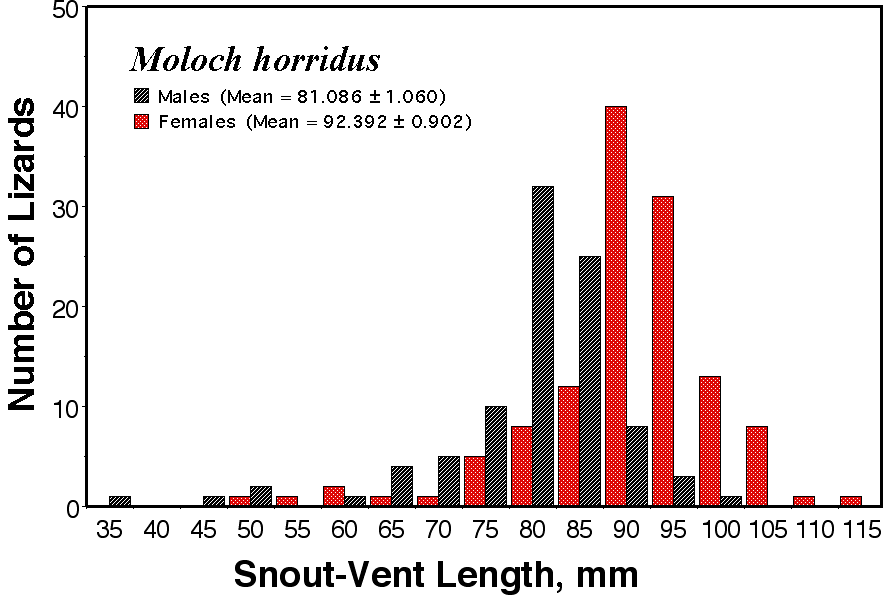 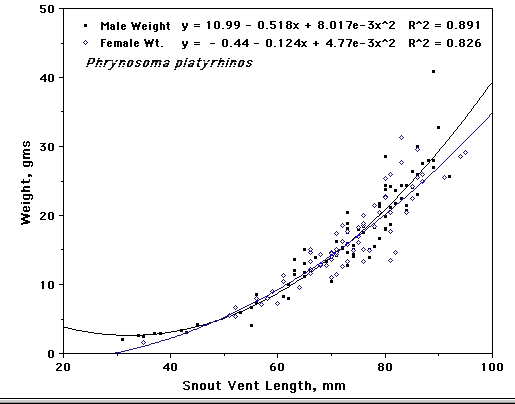
Weight plotted against snout-vent length for male and female Phrynosoma platyrinos 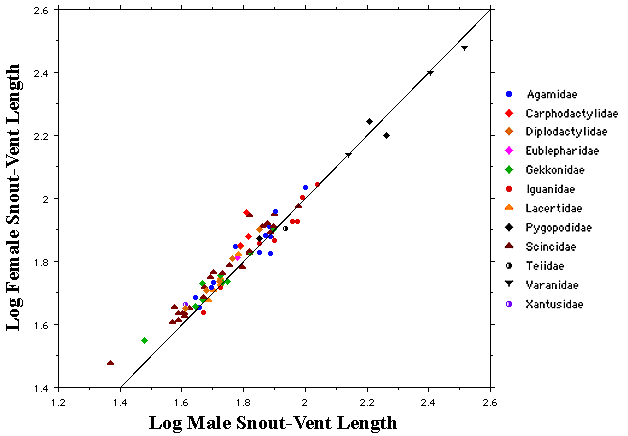
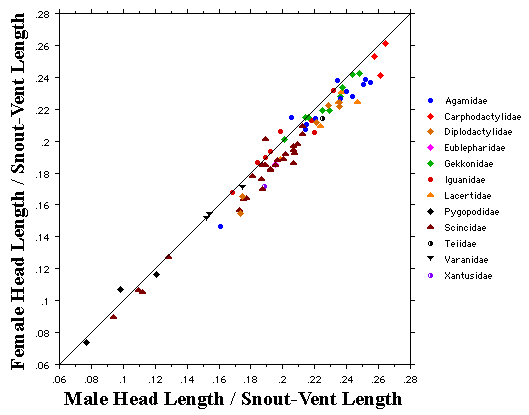 As a result, head size dimorphisms are the rule (Vitt and Cooper 1985). In two species of varanids, Varanus eremius and V. gouldii, heads of males are proportionately larger than those of females (Pianka 1994). This is shown for the Kalahari arboreal skink Trachylepis (formerly Mabuya) striata and for the Australian pygmy monitors Varanus eremius and Varanus brevicauda in the next three plots. Males have proportionately larger heads than females in many Australian skinks as well, including Cryptoblepharus buchananii, Ctenotus leonhardii and Ctenotus quattuordecimlineatus. 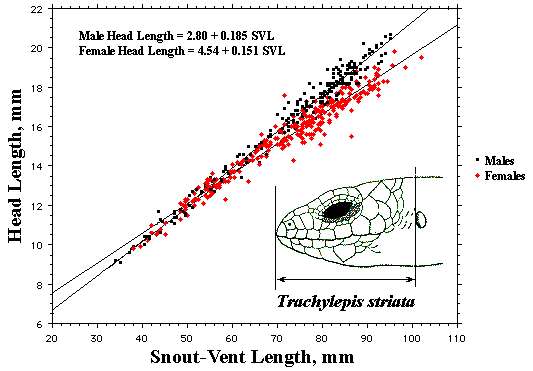 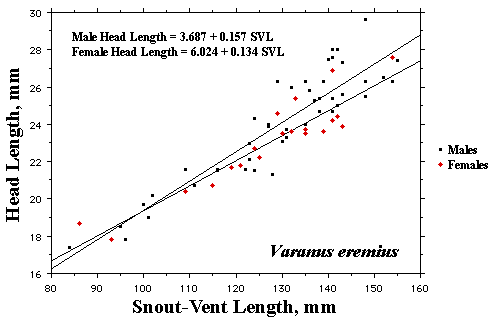 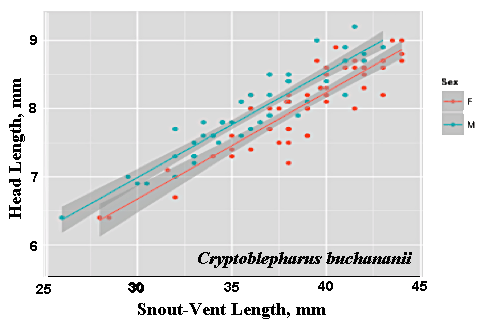 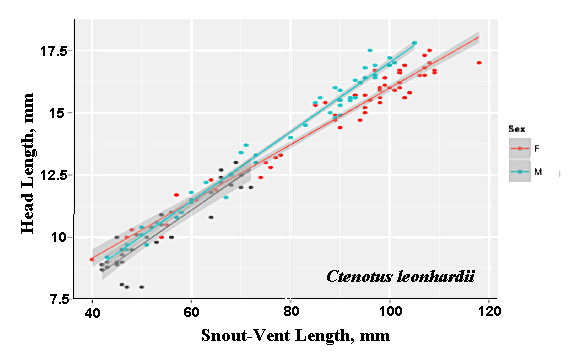 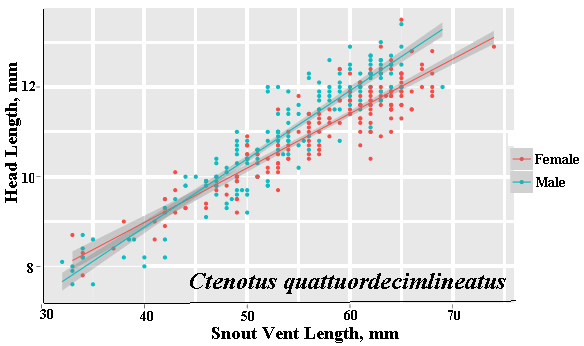 In many lizard species, such as Varanus brevicauda and Trachylepis striata, males have longer tails than females. 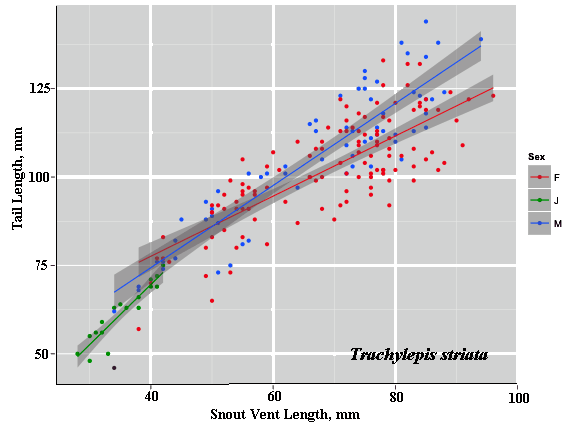 Relative hind leg length is positively correlated with the frequency of use of open space among Australian skinks and geckos (Pianka 1969, Pianka and Pianka 1976). Presumably, longer hind legs confer faster running speeds for retreat to cover. Conversely, shorter hind legs are favored in species that spend the majority of time in dense vegetation allowing them to move easily (sometimes called "grass swimming"). Some male varanids have longer hind legs than females (Pianka 1994), as illustrated below for the Australian pygmy monitor Varanus eremius. 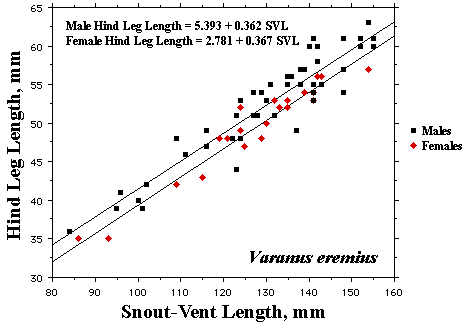 Reproductive Biology As noted above, clutch size increases with female body size in most lizard species, but all geckos have a fixed clutch size of only one or two eggs. Female investment in reproduction, termed reproductive effort, as measured by relative clutch mass (RCM) is variable among species (Reproductive Tactics). In thorny devils, Moloch horridus, an agamid, fat body cycles differ between the sexes. Females build up fat reserves during late summer-autumn, whereas males do so during late spring and early summer. Females deplete their fat reserves during spring when they invest in yolking up large clutches of eggs, whereas during this same time period male fat reserves increase. Female fat bodies are enlarged during late autumn, when mating takes place, whereas male fat bodies are considerably smaller during this season. 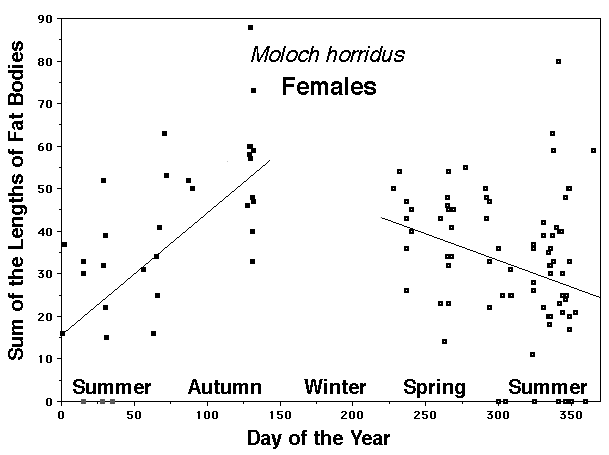
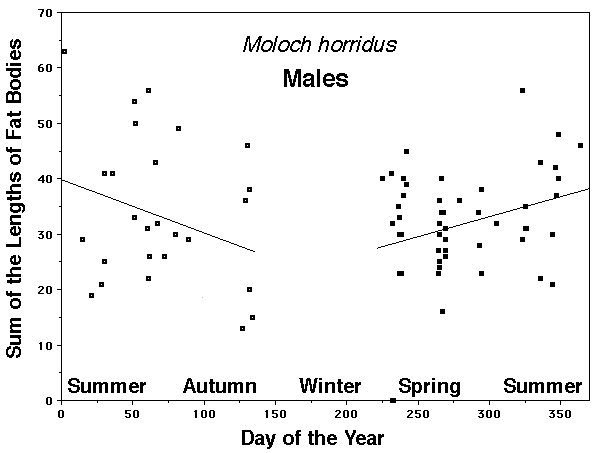
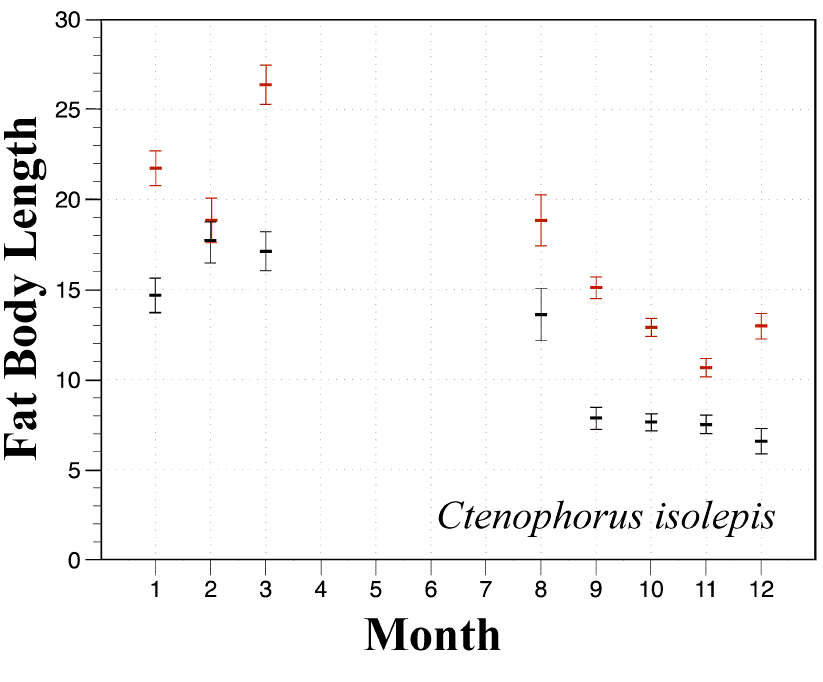
In a related agamid Pogona minor, mating takes place in the spring and male and female fat body cycles are synchronized. In another agamid Ctenophorus isolepis, fat bodies of females (red) are significantly larger than those of males (black) in all sampled months except February. 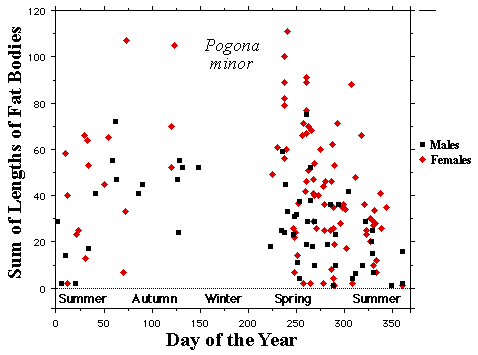
In the varanid V. tristis, fat bodies of both males and females are enlarged during spring when mating takes place. Male fat bodies and testes diminish in size during summer and begin to build again during autumn. Dietary Differences In most species, males and females consume prey in similar proportions and exhibit relatively little difference in dietary niche breadths. However, dietary differences do occur between the sexes in a few species, probably stemming from differences in energy requirements and foraging behaviors. Dietary niche breadths were estimated using the inverse of Simpson's (1949) index of diversity, 1/Σpi2, where pi is the proportion of the diet by volume of prey resource state i. Sexual dimorphisms in diets do occur in some species such as in the North American iguanian Phrynosoma platyrhinos. These horned lizards are ant specialists with narrow diets, but males consume more ants by volume than females (62.7% versus 52.9%) and females eat more beetles than males (26.9% vs. 20%). As a result of more equitable consumption of prey, the dietary niche of females is broader than that of males (2.76 vs. 2.26). Sample sizes consist of 74 females and 96 males. Male and female North American iguanians in the species Uta stansburiana eat beetles and ants in similar proportions (about 21-23% and 14-15%, respectively), but the sexes differ in their consumption of termites and grasshoppers: by volume, females eat termites 16.5% and grasshoppers 15.4% whereas males eat more termites (28.2%) but fewer grasshoppers (11.3%). Resulting dietary niche breadth of females is greater than that for males (7.12, N= 489 vs. 6.0, N = 471). In the Kalahari agamid Agama hispida, ants comprise about half the diet by volume in both sexes, but males eat more beetles than females (27.4% vs. 17%, N's = 191 and 155, respectively) whereas female Agama consume more termites than males (18.7% vs. 11.4%). Because of more equitable consumption of prey, dietary niche breadth of Agama females is greater (3.47) than it is for males (2.98). In the Kalahari skink Trachylepis occidentalis, dietary niche breadth of females is also greater (5.15, N = 105) than for males (3.81, N = 105). Females eat more locustids than males (17.6% vs. 5.2%) whereas males consume more beetles (43.8% vs. 33.5%) and termites (24.6 vs. 20%) than females. In Australian varanids, no sexual dimorphism in diet was evident in Varanus eremius, but in V. gouldii, males were more specialized than females (3.6 vs. 5.39, N's = 57 and 48). Male V. gouldii ate 49.2% vertebrates compared to only 36.1% in females. Both sexes ate centipedes in similar proportions, but females consumed greater amounts of beetles and scorpions than males (13.4% vs. 6.6% and 10.4% vs. 6.1%). In some other species, males have broader diets than females. For example, in the Kalahari gecko Chondrodactylus angulifer, dietary niche breadth of males is broader (4.40, N = 220) than it is for females (3.26, N = 187). Males consume fewer termites (40.7% vs. 51.5% in females) but more beetles and scorpions than females (12% and 13.5% vs. 9.7% and 7.2%). Female Chondrodactylus ate more crickets than males (13.7% vs. 11.2%). In the nocturnal Australian skink Egernia striata (now Liopholis, Gardner et al. 2008), dietary niche breadth based on stomach contents of 147 females is 3.27, whereas a sample of 176 males had a considerably broader dietary niche breadth of 6.32. Females consumed 52.5% termites by volume whereas males sampled prey types more equitably and ate only 33.7% termites by volume. Males and females consumed similar proportions of ants and beetles by volume (10% vs. 9% and 10.1% vs. 11.4%, respectively), but males ate greater amounts of spiders (9.9% vs. 5%) and vegetation (6.1% vs. 3.8%) than females. In the diurnal Australian skink Ctenotus quattuordecimlineatus, males and females consume locustids and hemipterans in similar proportions (15.5% vs. 16.2% and 9.7% vs. 10.2%), but males eat more spiders (14.1% vs. 8.1%) and fewer termites than females (25.7% vs. 36.1%) leading to higher prey equitability and broader dietary niches in males (7.65, N = 589) than in females (5.51, N = 455). Phylogenetic Constraints A combined phylogenetic tree was constructed based on recent references (Gamble et al. 2012, Hugall et al. 2008, Rabosky et al. 2007, Sites et al. 2011, Zheng and Weins 2016). 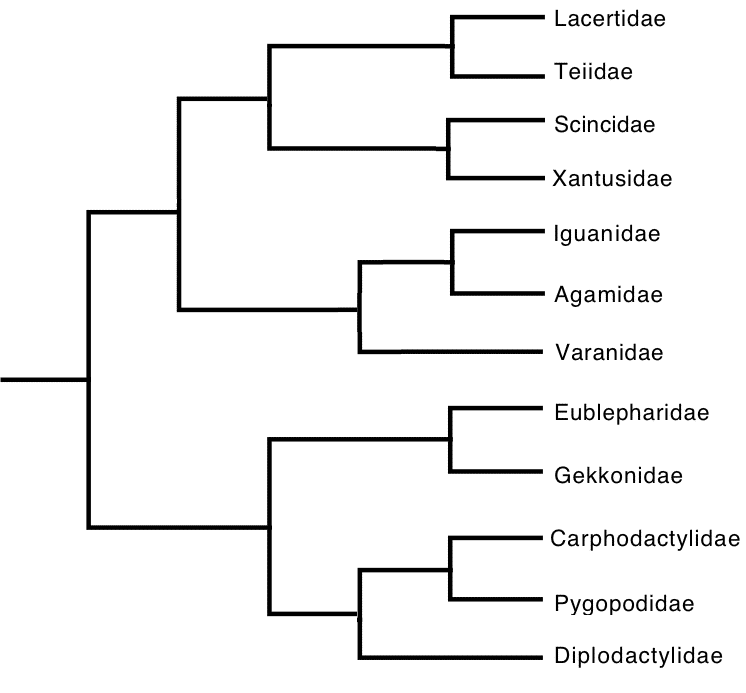 Discussion References Gamble, T, E. Greenbaum, T. R. Jackman, A. P. Russell, and A. M. Bauer. 2012. Repeated Origin and Loss of Adhesive Toepads in Geckos. PLoS ONE 7(6): e39429. Gardner, M.G., Hugall, A.F., Donnellan, S. C., Hutchinson, M. N., and Foster, R. 2008. Molecular systematics of social skinks: phylogeny and taxonomy of the Egernia group (Reptilia: Scincidae). Zool. J. Linn. Soc. 154: 781-794. Huey, R. B. and E. R. Pianka. 2007. Lizard thermal biology: do genders differ? Amer. Natur. 170: 473-478. Download pdf. Hugall, A. F., R. Foster, M. Hutchinson, and M. S. V. Lee. 2008. Phylogeny of Australasian agamid lizards based on nuclear and mitochondrial genes: implications for morphological evolution and biogeography. Biological Journal of the Linnean Society 93: 343-358. Pianka, E. R. 1969. Sympatry of desert lizards (Ctenotus) in Western Australia. Ecology 50: 1012-1030. Download pdf. Pianka, E. R. 1994. Comparative ecology of Varanus in the Great Victoria desert. Australian Journal of Ecology 19: 395-408. Download pdf. Pianka, E. R. 1997. Australia's thorny devil. Reptiles 5 (11): 14-23. Download pdf. Pianka, E. R. and W. S. Parker. 1975. Age-specific reproductive tactics. American Naturalist 109: 453-464. Download pdf. Pianka, E. R. and H. D. Pianka. 1970. The ecology of Moloch horridus (Lacertilia: Agamidae) in Western Australia. Copeia 1970: 90-103. Download pdf. Pianka, E. R. and H. D. Pianka. 1976. Comparative ecology of twelve species of nocturnal lizards (Gekkonidae) in the Western Australian desert. Copeia 1976: 125-142. Download pdf. Pianka, G. A., E. R. Pianka, and G. G. Thompson. 1996. Egg laying by thorny devils under natural conditions in the Great Victoria desert. J. Roy. Soc. Western Australia 79: 195-197. Download pdf. Pianka, G. A., E. R. Pianka, and G. G. Thompson. 1998. Natural history of thorny devils Moloch horridus (Lacertilia: Agamidae) in the Great Victoria desert. J. Royal Society of Western Australia 81: 183-190. Download pdf. Pyron, R. A., F. T. Burbrink and J. J. Wiens. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology 13,93: 1-53. Rabosky, D. L., S. C. Donnellan, A. L. Talaba, and I. J. Lovette. 2007. Exceptional among-lineage variation in diversification rates during the radiation of Australia's most diverse vertebrate clade. Proceedings of the Royal Society B 274: 2915-2923. Shine, R. 1989. Ecological Causes for the Evolution of Sexual Dimorphism: A Review of the Evidence. Quarterly Review of Biology 64: 419-461. Sites, J. W., T. W. Reeder, and J. J. Wiens. 2011. Phylogenetic Insights on Evolutionary Novelties in Lizards and Snakes: Sex, Birth, Bodies, Niches, and Venom. Ann. Rev. Ecol. Evol. Syst. 42: 227-244. Simpson, E. H. 1949. Measurement of diversity. Nature 163: 688. Thompson, G. G., E. R. Pianka, and M. de Boer. 1999. Body temperatures of an arboreal monitor lizard, Varanus tristis (Squamata: Varanidae) during the breeding season. Amphibia-Reptilia 20: 82-88. Download pdf. Thompson, G. G. and E. R. Pianka. 1999. Reproductive ecology of the black-headed goanna Varanus tristis (Squamata: Varanidae). J. Royal Society of Western Australia 82: 27-31. Download pdf. Thompson, G. G., M. de Boer, and E. R. Pianka. 1999. Activity areas and daily movements of an arboreal monitor lizard, Varanus tristis (Squamata: Varanidae) during the breeding season. Australian Journal of Ecology 24: 117-122. Download pdf. Verwaijen, D. and R. Van Damme. 2008. Foraging Mode and Its Flexibility in Lacertid Lizards From Europe. J. Herpetology, 42(1):124-133. Vitt, L. J., and W. E. Cooper, Jr. 1985. The relationship between reproduction and lipid cycling in the skink Eumeces laticeps with comments on brooding ecology. Herpetologica 41: 419-432. Zheng, Y. and J. J. Weins. 2016. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Molecular Phylogenetics and Evolution 94, Part B, Pages 537-547. |