|
© Eric R. Pianka How organisms go about their daily activities and how they use their environments are of great interest to ecologists. The various ways in which organisms conform to the conditions imposed upon them by their surroundings are known as adaptations. All aspects of an animal's behavior are important -- natural history includes spatial and temporal patterns of activity, thermoregulation, foraging behavior, diet, courtship, mating, reproduction, as well as escape from predators. Use of Time and Space: Thermal RelationsAnimals that maintain relatively constant internal body temperatures are known as homeotherms, whereas those whose temperature varies widely from time to time, often approximating the temperature of their immediate environment, are called poikilotherms. A related pair of useful terms are sometimes confused with these two terms. An ectotherm obtains its heat from its external environment, whereas an endotherm produces most of its own heat internally by means of oxidative metabolism. All plants, and the vast majority of animals, are ectothermic; the only continuously endothermic animals are birds and mammals (some of these become ectothermic at times). Certain poikilotherms, including large snakes and large lizards, are at times at least partially endothermic, too. Many ectothermic lizards actually regulate their body temperatures very precisely during periods of activity by appropriate behavioral means, thus achieving a degree of homeothermy. An active desert lizard may have a body temperature every bit as high as that of a bird or mammal (the layperson's misleading terms "warm-blooded" and "cold-blooded" should be abandoned). Lizards constitute an extremely conspicuous element of the vertebrate faunas of most deserts, especially warmer ones. Indeed, the Australian mammalogist H. H. Finlayson (1943) referred to the vast interior deserts of Australia as "a land of lizards." Ectothermy facilitates metabolic inactivity, allowing lizards to capitalize on scant, unpredictable food supplies. Moreover, along with other ectotherms, lizards are low-energy animals. One day's food supply for a small bird will last a lizard of the same weight for a month or more. Ectothermy thus has distinct advantages over endothermy under the harsh and unpredictable conditions that prevail in deserts. By means of this thermal tactic, lizards can conserve water and energy by becoming inactive during the heat of midday, during resource shortages, or whenever difficult physical conditions occur (such as during heat waves or droughts). Birds and mammals must weather these inhospitable periods at a substantially higher metabolic cost. Hence ectothermy confers a competitive advantage on lizards over endotherms in desert environments. In temperate zones, hot arid regions typically support rich lizard faunas, whereas cooler forested areas have many fewer lizard species and individuals. Lizards can enjoy the benefits of a high metabolic rate during relatively brief periods when conditions are appropriate for activity, yet still become inactive during adverse conditions. By facilitating metabolic inactivity on both a daily and a seasonal basis, poikilothermy allows lizards to capitalize on unpredictable food supplies. Moreover, lizards can effectively reduce temporal heterogeneity by retreating underground, becoming inactive, and lowering their metabolic rate during harsh periods (some desert rodents estivate when food or water is in short supply). Poikilothermy may well contribute to the apparent relative success of lizards over birds and mammals in arid regions. Temperate zone forests and grasslands are probably too shady and too cold for ectothermic lizards to be very successful because these animals depend on basking to reach body temperatures high enough for activity; birds and mammals, in contrast, do quite well in such areas partly because of their endothermy. Lizards do well in warm tropical forests, however. A spectacular example of the capacity of lizards to go dormant took place in Arizona during the 1960s. An instant "Sun City" was built in the Sonoran desert. This small town had been in place for several years without appreciable rainfall, but the local residents had planted lawns and trees which were irrigated with ground water pumped up from the water table. When massive August rains finally fell, Gila monsters began popping up out of the ground in people's yards -- these lizards had been buried underground, inactive, living off the stored fat in their tails during the time that the city was being built. When averaged over a long enough period of time, the heat gained by an animal must be exactly balanced by the heat lost back to its environment; otherwise the animal would either warm up or cool off. Many different pathways of heat gains and heat losses exist. Balancing a heat budget requires very different adaptations under varying environmental conditions. At different times of day, ambient thermal conditions may change from being too cold to being too warm for a particular animal's optimal performance. Animals living in hot deserts must avoid overheating by being able to minimize heat loads and to dissipate heat efficiently; in contrast, those that live in colder places, such as at high altitudes and high latitudes, must be adept at acquiring, and retaining heat. Environmental temperatures fluctuate in characteristic ways at different places over the earth's surface, both daily and seasonally. In the absence of a long-term warming or cooling trend, environmental temperatures at any given place remain roughly constant when averaged over an entire annual cycle. The range in temperature within a year is much greater at high latitudes than it is nearer the equator. An animal could balance its annual heat budget by being entirely passive and simply allowing its temperature to mirror that of its environment. Such a passive thermoregulator is known as a thermoconformer. Of course, it is also an ectotherm. Another extreme tactic is to maintain an absolutely constant body temperature by physiological, and/or behavioral means, dissipating (or avoiding) excess bodily heat during warm periods but retaining (or gaining) heat during cooler periods. Such organisms that carefully regulate their internal temperatures are thermoregulators. Both endotherms and ectotherms regulate their body temperatures. There is a continuum between the two extremes of perfect conformity and perfect regulation. Regulation is never perfect. Because thermoregulation clearly has costs and risks as well as profits, an optimal level of regulation depends on the precise form of the constraints, and on the interactions between costs and benefits arising from a particular ecological situation. Thermoregulation often involves both physiological and behavioral adjustments; as an example of the latter, consider a typical terrestrial diurnal desert lizard. During the early morning, when ambient temperatures are low, such a lizard locates itself in warmer microclimates of the environmental thermal mosaic (for example, in a small depression in the open or on a sunny tree trunk), basking in the sun with its body as perpendicular as possible to the sun's rays, thereby maximizing heat gained. With the daily march of temperature, ambient temperatures quickly rise, and the lizard seeks cooler shady microhabitats. Individuals of some species retreat into burrows as temperatures rise; others climb up off the ground into cooler air and orient themselves facing into the sun's rays, thereby reducing heat load. Many lizards change colors, and thus their heat-reflective properties; they turn dark, and heat absorbent, at colder times of day but pale, and heat reflective, at hotter times. Such adjustments allow individual lizards to be active over a longer period of time than they could be if they conformed passively to ambient thermal conditions; presumably, these lizards are also more effective competitors and better able to elude predators as a result of such thermoregulatory behaviors. Thermoregulation in lizards is not as simple as it might first seem, for it encompasses a wide diversity of very different thermoregulatory tactics among species, ranging from ectothermic poikilothermy to, and including, ectothermic homeothermy. Even a casual observer quickly notices that various species of desert lizards differ markedly in their times and places of activity. Some species are active early in the morning, but others do not emerge until late morning or midday. Most geckos and pygopodids, and some Australian skinks, are nocturnal. Certain species are climbers, others subterranean, while still others are strictly surface dwellers. Among the latter, some tend to be found in open areas, whereas others frequent the edges of vegetation. Thermal relations of active lizards vary widely among species and are profoundly influenced by their spatial and temporal patterns of activity. Body temperatures of some diurnal species average 38°C or higher, whereas those of nocturnal species are typically in the midtwenties, closely paralleling ambient air temperatures. Interesting differences between species also occur in the variance in body temperature as well as in the relationship between body temperatures and air temperatures. For example, among North American desert lizards, two arboreal species (Urosaurus graciosus and Sceloporus magister) display narrower variances in body temperature than do terrestrial species occurring on the same study sites. Presumably, arboreal habits often facilitate efficient, economic, and rather precise thermoregulation. Climbing lizards have only to shift position slightly to be in the sun or shade, or on a warmer or cooler substrate, and normally do not move through a diverse thermal environment. Moreover, arboreal lizards need not expend energy making long runs as do most ground-dwellers, and thus climbing species do not raise their body temperatures metabolically to as great an extent as do terrestrial lizards. Such differences in temporal patterns of activity, the use of space, and body temperature relationships are hardly independent. Rather, they complexly constrain one another, sometimes in intricate and obscure ways. For example, thermal conditions associated with particular microhabitats change in characteristic ways in time: a choice basking site at one time of day becomes an inhospitable hot spot at another time. Perches of arboreal lizards receive full sun early and late in the day when ambient air temperatures tend to be low, and basking is therefore desirable, but these same tree trunks are shady and cool during the heat of midday when heat avoidance behavior becomes necessary. In contrast, the fraction of the ground's surface in the sun is low early and late when shadows are long, but reaches a maximum at midday. Terrestrial heliothermic lizards may thus experience a shortage of suitable basking sites early and late in the day; moreover, during the heat of the day, their movements through relatively extensive patches of open sun can be severely curtailed. Hence ground-dwelling lizards encounter fundamentally different, and more difficult, thermal challenges than do climbing species. Radiation, convection, and conduction are the most important means of heat exchange for the majority of diurnal lizards, although the thermal background in which these processes occur is strongly influenced by prevailing air temperatures. Ambient air temperatures are critical to nocturnal lizards as well as to certain very cryptic diurnal species. In an analysis of the costs and benefits of lizard thermoregulatory strategies, Huey and Slatkin (1976) identified the slope of the regression of body temperature against ambient environmental temperature as a useful indicator (in this case, an inverse measure) of the degree of passivity in regulation of body temperature. On such a plot of active body temperature versus ambient temperature, a slope of one indicates true poikilothermy or totally passive thermoconformity (a perfect correlation between air temperature and body temperature results), whereas a slope of zero reflects the other extreme of perfect thermoregulation. Lizards span this entire thermoregulation spectrum: among active diurnal heliothermic species, regressions of body temperature on air temperature are fairly flat (for several species, including some quite small ones, slopes do not differ significantly from zero); among nocturnal species, slopes of similar plots are typically closer to unity. Various other species (nocturnal, diurnal, and crepuscular), particularly Australian ones, are intermediate, filling in this continuum of thermoregulatory tactics. A straight line can be represented as a single point in the coordinates of slope versus intercept; these two parameters are plotted for linear regressions of body temperatures on air temperatures among some 82 species of lizards in the above Figure. Each data point represents the least-squares linear regression of body temperature against air temperature for a given species of desert lizard. Interestingly enough, these data points fall on another, transcendent, straight line. The position of any particular species along this spectrum reflects a great deal about its complex activities in space and time. The line plotted offers a potent linear dimension on which various species can be placed in attempts to formulate general schemes of lizard ecology. Various other ecological parameters, including reproductive tactics, can be mapped onto this emergent spatial-temporal axis. The intriguing "intercept" of the intercepts (38.8°C) approximates the point of intersection of all 82 regression lines and presumably represents an innate design constraint imposed by lizard physiology and metabolism. It is no accident that this value also corresponds more or less to our own body temperatures, and indeed, to those of all mammals! Most, but not all, lizards have a small light sensitive third eye mid-way between their two lateral eyes located beneath the parietal scale on top of their head. This third eye is thought to serve as a dosimeter of solar radiation, and sensory input from it is integrated with hormones to control the daily cycle of activity or circadian rhythm (i.e., the lizard's "biological clock"). Not all reptiles possess the parietal eye -- it is lacking in turtles, snakes, crocodilians, and is not present in several families of lizards (Teiidae, Gekkonidae, and Helodermatidae). Birds and mammals do not have this third eye, either. Feeding EcologyIn an environment with a scant food supply, a consumer cannot afford to bypass many inferior prey items because mean search time per item encountered is long, and expectation of prey encounter is low. In such an environment, a broad diet maximizes returns per unit expenditure, favoring generalization. In a food-rich environment, however, search time per item is reduced since a foraging animal encounters numerous potential prey items; under such circumstances, substandard prey items can be bypassed economically because the expectation of finding a superior item in the near future is high. Hence rich food supplies are expected to favor selective foraging and to lead to narrow diets. In reality, of course, food abundances are ever changing, both spatially and temporally. Certain species of lizards are dietary specialists, eating only a very narrow range of prey items. For example, the Australian agamid Moloch horridus eats essentially nothing except ants, mostly of a single species of Iridomyrmex (North American horned lizards, genus Phrynosoma, are also ant specialists). Other lizard species are termite specialists, including the Australian nocturnal geckos Diplodactylus conspicillatus and Rhynchoedura ornata, plus some species of diurnal Ctenotus comb-eared skinks, as well as the Kalahari lacertid Heliobolus lugubris and fossorial (subterranean) Typhlosaurus skinks. Even though these species eat virtually nothing but isoptera, specialization on termites and ants is economically feasible because these social insects normally occur in a clumped spatial distribution, and hence constitute a concentrated source of food. Still other lizard species, though not quite so specialized, also have narrow diets. For example, the beautiful Kalahari lacertid Nucras tessellata and the Australian pygopodid Pygopus nigriceps both consume considerably more scorpions than do other lizard species. Nucras forages widely to capture these large arachnids by day in their diurnal retreats, whereas the nocturnal Pygopus sits and waits for scorpions at night above ground during the latter's normal period of activity. While scorpions are solitary prey items, they are extremely large and nutritious, presumably facilitating evolution of dietary specialization. For similar reasons, specialization on other lizards as food items has evolved in the North American Gambelia wislizeni as well as among most Australian Varanus. Other species of lizards eat a considerably wider variety of foods. Dietary diversity also varies within species from time to time and from place to place as the composition of diets changes with opportunistic feeding in response to fluctuating prey abundances and availabilities. However, the consistency of lizard diets over space and time is fairly remarkable, suggesting a profound impact of microhabitat utilization, foraging mode, as well as various anatomical, historical (phylogenetic), and behavioral constraints. Another, more extreme, example of this phenomenon occurs after heavy summer rains when termites send out their winged reproductives in great abundance, and virtually every species of lizard eats nothing but termites (even lizard species that normally never consume termites). During such fleeting moments of great prey abundance, competition for food is weak, and dietary overlap among members of a desert lizard fauna is sometimes nearly complete. Biologically significant variation occurs between species in utilization of certain relatively minor food categories: for example, in the diets of climbing lizard species, hemiptera-homoptera and mantids-phasmids as well as various flying insects (wasps, Diptera, and Lepidoptera) tend to be better represented than they are among terrestrial species. Likewise, geckos tend to consume more nocturnal arthropods (scorpions, crickets, roaches, and moths) than do most diurnal species (however, certain diurnal lizards, such as Nucras mentioned above, do capture nocturnal prey in their diurnal retreats). Such prey items are thus indicators of spatial and temporal patterns of activity. Only a relatively few food types dominate diets of desert lizards. Prey resource spectra are broadly similar between the three continents, although notable quantitative differences occur. In North America, the seven most important food types (totaling 84 percent), in decreasing order by volumetric importance, are: beetles, termites, insect larvae, grasshoppers plus crickets, ants, plant materials, and vertebrates. In the Kalahari, just three food categories far outweigh all others (total 71 percent): termites, beetles, and ants. In Australia, the five most important categories (total 77 percent, in decreasing order) are: vertebrates, termites, ants, grasshoppers plus crickets, and beetles. Three categories, termites, beetles, and ants, constitute major prey items in all three continental desert-lizard systems. Termites assume a disproportionate role in the Kalahari, as do vertebrate foods in Australia (largely a reflection of the diets of varanids). Many predators attack their prey from ambush, but others usually hunt while on the move. These two modes of foraging have been called the "sit-and-wait" versus the "widely foraging" tactic, or "ambush" versus "active", respectively. Of course, this dichotomy is somewhat artificial, although numerous animal groups seem to fall rather naturally into one category or the other. Members of most lizard families typically exploit one or the other of these two modes of foraging; thus iguanians, agamids, and geckos primarily sit and wait for their prey, whereas teiids and most skinks forage widely. Lacertids, however, use both modes of foraging, even within closely related species. Certain dietary differences are associated with this apparent dichotomy in foraging tactics. Sit-and-wait predators rely largely on moving prey, whereas widely foraging predators encounter and consume non-moving types of prey items more frequently. For the sit-and-wait ambush tactic to pay off, prey must be relatively mobile, and prey density must be high (or predator energy requirements low). The success of the widely-foraging active foraging tactic is also influenced by prey mobility and prey density as well as by the predator's energetic requirements (which should usually be higher than those of sit-and-wait predators), but the searching abilities of the predator and the spatial distribution of its prey assume substantial importance. North American and Australian desert study sites support similar numbers of species of sit-and-wait foragers, whereas this mode of foraging is distinctly impoverished in the Kalahari. Markedly fewer species forage widely in western North America (only one species, a teiid) and in the Kalahari (an average of four species per site) than in the Australian deserts (the mean number of widely foraging species per area is ten, most of which are comb-eared skinks in the genus Ctenotus). Intercontinental comparisons of proportions of total species in various foraging modes are also instructive: a full 60 percent of North American lizard species are sit-and-wait foragers, compared to only 16 percent in the Kalahari, and 18 percent in Australia. Percentages of widely foraging species are 14 percent (North America), 27 percent (Kalahari), and 36 percent (Australia). Adaptive SuitesNow consider one of these dietary specialists in somewhat greater detail. North American desert horned lizards, Phrynosoma platyrhinos, are ant specialists, eating little else. Various features of their anatomy, behavior, diet, temporal pattern of activity, thermoregulation, and reproductive tactics can be profitably interrelated and interpreted to provide an integrated view of the ecology of these interesting lizards (Figure). 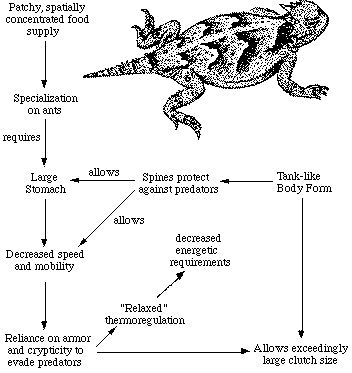
Ants are small, and contain much undigestible chitin, so that large numbers of them must be consumed. Hence an ant specialist must possess a large stomach for its body size. The stomach of this horned lizard averages about 13 percent of the animal's overall body mass, a substantially larger fraction than stomachs of other lizard species, even among herbivorous lizards (in six other sympatric North American lizard species, stomach volumes average only 6.4 percent of body weight; among eight Australian species selected as crude ecological counterparts, the ratio of stomach volume to body weight averages only 5.9 percent). Phrynosoma's large stomach requires a tank-like body form, reducing speed and decreasing the lizard's ability to escape from predators by movement. As a result, natural selection has favored a spiny body form and cryptic behavior rather than a sleek body and rapid movement to cover, as in the majority of other lizards. Long periods of exposure while foraging in the open presumably increase risks of predation. A reluctance to move, even when actually threatened by a potential predator, is advantageous under such circumstances. Movement might attract the predator's attention and negate the advantage of concealing coloration and contour. Such decreased movement contributes to the observed high variability in body temperature of Phrynosoma platyrhinos, which is significantly greater than that of all other sympatric species of lizards. Wide fluctuations in horned lizard body temperatures under natural conditions presumably reflect both their long activity period, and perhaps their reduced movements into or out of the sun, and shade (most active Phrynosoma are in the open sun when first encountered). A consequence is that more time is made available for activities such as eating (foraging ant eaters must spend considerable time feeding). To make use of this patchy and spatially concentrated, but at the same time not overly nutritious, food supply, Phrynosoma platyrhinos has evolved a unique constellation of adaptations (an "adaptive suite") that includes its large stomach, spiny tank-like body form, expanded period of activity, and "relaxed" thermoregulation. Another spin-off of the Phrynosoma adaptive suite concerns their extraordinarily high investment in reproduction. Females of some species of horned lizards devote as much as 35 percent of their body weight to production of a very large clutch of eggs. This is presumably a simple and direct consequence of their robust body form: lizards that must be able to move rapidly to escape from predators would hardly be expected to weight themselves down with eggs to the same extent as animals like horned lizards that rely almost entirely upon spines and camouflage to avoid their enemies. Reproductive TacticsMost lizards lay eggs, but some species retain their eggs internally and give birth to living young. Live-bearing has arisen repeatedly among squamates (lizards and their close relatives snakes), even multiple times within a single genus. Live-bearing and egg retention is especially prevalent in cooler regions at high elevations and high latitudes. Average clutch size varies from one to nearly fifty among different species of lizards. Some species reproduce only once every second or third year, others but once each year, and still others lay two or more clutches each year. Lizards that lay only one egg or give birth to a single young include the American iguanid genus Anolis, the Kalahari skink Typhlosaurus gariepensis, and the geckos Gehyra variegata, G. purpurascens, and Ptenopus garrulus. Clutch size is fixed at one or two eggs in certain families (Geckos, Pygopodids) and genera (Anolis). Across all species, the modal clutch size among lizards is two eggs. In the Kalahari agamid Agama aculeata , clutch size averages 13. Clutch sizes in certain horned lizards are still larger, averaging 24.3 in the American iguanid Phrynosoma cornutum (the Texas horned lizard). One of the most fecund lizards is Ctenosaura pectinata, one female of which had 49 eggs in her oviducts. Substantial spatial and temporal variation in clutch size also exists within species. In the double-clutched Australian agamid species Ctenophorus isolepis, the size of 67 first clutches (August-December) averaged 3.0 eggs, whereas the mean of 41 second clutches (January-February) was 3.9. Females increase in size during the season, and as in many lizards, larger females tend to lay larger clutches. Females also appear to invest relatively more on their second clutches than they do on their first clutch: among 25 first clutches, clutch volumes average only 11 percent of female weight, but in 15 second clutches, the average is 15 percent. Changes in fecundity with fluctuations in food supplies and local conditions from year to year or location to location have also frequently been observed. In many species, females tend to lay larger clutches in years with above-average precipitation, and presumably ample food supplies. Clutch or litter mass (either weight or volume), expressed as a fraction of a female's total body weight, also known as "relative clutch mass," ranges from as little as 4-5 percent in some species to as much as 20-30 percent in others. Clutch weights tend to be particularly high in some of the North American horned lizards (genus Phrynosoma). Ratios of clutch or litter weight to female body weight correlate strongly with various energetic measures, and can be used as crude indices of a female's instantaneous investment in current reproduction. In addition to clutch size, and female total investment in reproduction, the size (or weight) of an individual oviductal egg or newborn progeny also varies widely among lizards from as little as 1-2 percent in some species to a full 17 percent in the live-bearing Kalahari fossorial skink Typhlosaurus gariepensis. Such expenditures per progeny are inverse measures of the extent to which a juvenile lizard must grow to reach adulthood. Any two parties to this triad (clutch size, female reproductive investment, and expenditure per progeny) uniquely determine the third; however, forces of natural selection molding each differ substantially. Clutch or litter weight presumably reflects an adult female's best current investment tactic in a given environment at a particular instant in time, whereas expenditure on any given individual progeny is more closely attuned to the average environment encountered by a juvenile. Clutch or litter size is thus the direct result of the interaction between an optimal parental reproductive tactic and an optimal juvenile size (clutch size is simply the ratio of the former divided by the latter). Two lizard species with the highest expenditures per progeny, Typhlosaurus gariepensis and T. lineatus, almost certainly experience intense competition: (1) these live-bearing, subterranean Kalahari skinks exist at very high population densities, (2) individuals are long-lived with delayed maturity, (3) litter sizes are extremely small (means of 1.0, and 1.5, respectively), and (4) females very likely reproduce only biennially. These two Kalahari fossorial skinks are also both extreme food specialists, eating termites to the virtual exclusion of all other prey. The exceedingly high expenditure per progeny of Typhlosaurus may well be necessary to confer newborn animals with competitive ability sufficient to establish themselves in the highly competitive underground environment. Limited evidence indicates that investment per progeny is indeed responsive to, and indicative of, a lizard's competitive environment. Thus, in Typhlosaurus lineatus, offspring are significantly heavier (and expenditure per progeny significantly greater) where this species occurs in sympatry with T. gariepensis as compared with allopatric populations. Other food-specialized species seem also to encounter intense competition. Among Australian geckos, species with relatively restricted termite diets tend to lay comparatively larger eggs, and hence have higher expenditures per progeny than do those with more catholic diets. Escape from PredatorsTails of many, but by no means all, lizards break off easily (indeed, some species can actually lose their tails voluntarily with minimal external force in a process known as autotomy). Freshly dismembered tails or pieces thereof typically thrash around wildly, attracting a predator's attention while the recent owner quietly slips away unnoticed. Certain small predators, such as the pygmy varanids Varanus gilleni and V. caudolineatus, actually "harvest" the exceedingly fragile tails of geckos that are too large to subdue intact. Some skinks, including many Ctenotus, return to the site where their tail was lost and swallow the remains of their own tail! Few, if any, other vertebrates display auto-amputation and self-cannibalism. Many such lizards possess special adaptations for tail loss, including weak fracture planes within each tail vertebra, muscular attachments that facilitate autotomy and tail movement after dismemberment, as well as mechanisms for rapidly closing off blood vessels and promoting healing. Losing its tail has surprisingly little effect on a lizard, as individuals often resume basking and foraging within minutes, as if nothing had happened. In such lizard species, of course, tails are quickly regenerated from the stub. Regrown tails are occasionally almost indistinguishable from the original externally, but their internal support structure is cartilaginous rather than bony. Not all lizard tails are easily broken, however. Whereas most iguanines have fragile tails, their close relatives the agamids generally do not. Tails of varanids and of true chameleons do not break easily either. Lizards with such tough tails usually cannot regenerate a very complete tail if their original should happen to be lost. The evolutionary bases for these differences, sometimes between fairly closely related groups of lizards, are evasive and merit further scrutiny. Lizard tails have diversified greatly, and they serve a wide variety of other functions for their possessors. Many climbing species, such as the Australian sandridge agamid Gemmatophora longirostris, have evolved extraordinarily long tails (three times the snout-vent length), which serve as effective counterbalances. Tail-removal experiments have shown that such long tails also enable lizards to raise their forelegs off the ground and to run on their hind legs alone (bipedality is a faster means of locomotion than tetrapodality). Prehensile tails are used as a fifth leg in climbing by other arboreal lizard species like some geckos (e.g., Strophurus elderi), and by the true chameleons such as Chameleo dilepis of the Kalahari. In several members of the Australian gekkonid genus Strophurus (S. ciliaris, S. elderi, and S. strophurus), glandular tails store and secrete a smelly noxious mucous. When disturbed, these lizards squirt out large amounts of sticky odoriferous goo. Surprisingly, tails of these geckos are fragile and easily shed (but quickly regenerated). One night, a small snake, and two geckos, including an S. ciliaris, fell into a pit trap: all were glued tightly together with S. ciliaris goo the next morning. A related Australian desert gecko, Diplodactylus conspicillatus, has a nonglandular but very short, stubby bony tail; these nocturnal termite specialists hide in the vertical shafts of abandoned spider holes during the day, where they point head downward, using their tails to block off these tunnels. Another Australian desert lizard with a similar yet different tail tactic is the climbing skink Egernia depressa. These lizards wedge themselves into tight crevices in mulga tree hollows (and rocks), blocking off the entrance with their strong and very spiny tails. Spinily armored tails are used by numerous other species of lizards in a similar fashion, including the Mexican iguanid Enyaliosaurus clarki and the Saharan agamid Uromastix acanthinurus. Members of a bizarre group of Australian lizards (knob-tailed geckos, genus Nephrurus) possess a unique round knob at the tip of their tails. These large nocturnal lizards eat big prey, including other species of geckos on occasion. Both sexes carry the curious knob, but its function remains a total mystery. Unlike most geckos, their tails are not very fragile. Nephrurus will stand their ground, and hiss and lunge with an open mouth in a sort of threat display. At such times, they also tend to arch their tails up over their backs, displaying the vivid white underside (their tails do break off if any pressure is applied). In many species of lizards (especially among juveniles), tails are brightly colored, and/or very conspicuous, evidently functioning to lure a potential predator's attack away from the more vulnerable, less dispensable parts of the animal. Thus, when approached or followed by a large animal, the zebra-tailed lizard of the western North American deserts, Callisaurus draconoides, curls its tail up over its hindquarters and back, exposing the bold black-and-white pattern underneath, and coyly wriggles its tail from side to side. If pursued farther, zebra-tailed lizards resort to extreme speed (estimated at up to 20-30 km/hr.), and long zigzag runs. An Australian desert skink, Ctenotus calurus, lashes and quivers its bright azure blue tail alongside its body continuously as it forages slowly through the open spaces between plants. Similarly, tiny Morethia butleri juveniles twitch their bright red tails as they move around in the litter beneath Eucalyptus trees. The potential of tail break frequency as an index to the intensity of predation on lizard populations was noted long ago by the legendary J. B. S. Haldane, and has since been used as a sort of bioassay to attempt to estimate the amount of predation, although this procedure has some problems and limitations. Efficient predators that leave no surviving prey obviously will not produce broken tails, but nevertheless may exert substantial predation pressures; broken and regenerated tails may therefore reflect lizard escape ability or predator inefficiency better than intensity of predation. In western North America, predator densities increase from north to south. Correlated with this latitudinal increase in predation, frequencies of broken and regenerated tails are higher at southern sites than at northern localities among four of the five widely distributed lizard species. In the well-studied desert whiptail species Aspidoscelis tigris, the frequency of broken tails decreases with latitude; moreover, diversity of predator escape behaviors utilized among members of various populations of other species of Aspidoscelis also increases with the frequency of broken and regenerated tails. A greater variety of escape tactics, a form of behavioral "aspect diversity," presumably reduces the ease with which predators can capture lizard prey. A nocturnal Australian skink, Eremiascincus richardsoni, very probably mimics the large centipede Scolpendra mortisans. Both are nocturnal, both frequent burrows, both are strongly banded with glossy yellow and black, and each has a reddish head. Of course, the centipede is poisonous with venomous claws on each of its many legs, as well as a toxic bite, whereas the skinks are quite defenseless. I have been told that, if a centipede ever begins to run across your bare skin, you should not try to sweep it off with your hand against the direction it is moving, as that will only assure that its poisonous claws will dig into your flesh. You can, apparently, sweep one off in the same direction that it is moving without getting poisoned. Think, before you react. Reflexes can get you into trouble!

|