Ecomorphology
Anatomical Correlates of Ecology (For an outline, click here)

Namib desert lacertid, Aporosaura anchietae, with duckbilled snout.
Fringed Toes and Shovel Noses
An open sandy desert poses severe problems for its inhabitants: (1) windblown sands are loose and provide little traction; (2) surface temperatures at midday rise to lethal levels; and (3) open sandy areas offer little food or shade or cover for evading predators. Even so, natural selection over eons of time has allowed lizards to cope fairly well with such sandy desert conditions. Subterranean lizards simply bypass most of the problems by staying underground, and actually benefit from the loose sand since underground locomotion is made easier. Burrowing is also facilitated by the evolution of a pointed, shovel-shaped head and a countersunk lower jaw, as well as by small appendages and muscular bodies and tails.
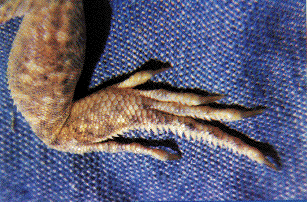
Toes of the North American fringe-toed lizard Uma scoparia.
During the hours shortly after sunrise, but before sand temperatures climb too high, diurnal lizards scurry about above ground in such sandy desert habitats. Sand specialized lizards provide one of the most striking examples of convergent evolution and ecological equivalence. Representatives of many different families of lizards scattered throughout the world's deserts have found a similar solution for getting better traction on loose sand: enlarged scales on the toes, or lamellae, have evolved independently in five different families of lizards: skinks, lacertids, phrynosomatids, agamids, and geckos (Luke 1986). A skink, appropriately dubbed the "sand fish," literally swims through sandy seas in search of insect food in the Sahara and other eastern deserts. These sandy desert regions also support lacertid lizards (Acanthodactylus) with fringed toes and shovel noses. Far away in the southern hemisphere, on the windblown dunes of the Namib desert of southwestern Africa, an independent lineage of lacertids has evolved a similar life form, Aporosaura anchietae, illustrated above.
In North America, this body form has been adopted by members of the iguanid genus Uma, which usually forage by waiting in the open and eat a fairly diverse diet of various insects, such as sand roaches, beetle larvae and other burrowing arthropods. They also listen intently for insects moving buried in the sand, and dig them up. Sometimes they dash, dig, and paw through a patch of sand and then watch the disturbed area for movements.
All of these lizards have flattened, duckbill-like, shovel-nosed snouts, which enable them to make remarkable "dives" into the sand even while running at full speed. The lizards then wriggle along under the surface, sometimes for over a meter. One must see such a sand diving act to appreciate fully its effectiveness as a disappearing act. Some Namib desert lizards discovered another solution to gain traction on powdery sands: frog-like webbing between the toes as seen in the gecko Palmatogecko.

Namib desert gecko, Palmatogecko rangeri.
Morphometrics
Population biologists have long appreciated that an animal's
morphology reflects its ecology (for a review, see Hespenheide 1973). Desert lizards are no exception. Species that spend a lot of time in the open away from cover tend to have longer hind legs, relative to their snout-vent length, than those that stay closer to safe retreats (Figure 11.1). Longer legs doubtlessly increase running speed and hence facilitate the use of open spaces. However, long-legged species move very clumsily through dense vegetation demonstrating that there is actually a premium on shorter legs for species that exploit such closed-in microhabitats. Terrestrial species also tend to have longer hind legs than arboreal species in Sceloporus (Lundelius 1957) and in Anolis (Collette 1961). Ananjeva (1977) found differences in limb proportions among five sympatric lacertids (genus Eremius), which proved to be correlated with their adaptations for climbing, burrowing, and other movements. Digging species typically possess larger front feet and more powerful forelegs than species that do not do much
digging with their front limbs. Fossorial species have reduced
appendages or lack them altogether.
Numerous other morphological correlates of the use of space exist.
Arboreal lizards are typically long-tailed and slender, with claws or
toe lamellae well suited for climbing. Indeed, the number of toe
lamellae, as well as their surface area, are thought to be intimately
related to climbing ability (Collette 1961, Hecht 1952). Among
nocturnal geckos, terrestrial species tend to have larger eyes than
arboreal species (Werner 1968; Pianka and Pianka 1976).
Head proportions and jaw length are often reasonably accurate
indicators of the size of a lizard's prey (Figures 11.2 and 11.3).
Lizard dentition also reflects more subtle aspects of diet, such as the
agility and hardness of prey (Hotton 1955).
An obvious hope is that such ecomorphological correlates will
ultimately enable ecological predictions based on anatomical data
(Ricklefs and Travis 1980). Another suggestion is that morphological
measures can be estimated more objectively than ecological parameters.
Still another idea is that morphometrics may represent average
long-term responses to selection and hence reflect environmental
conditions better than more direct measurements of the immediate
ecological milieu.
Van Valen (1965) postulated that morphologically variable species
should often be ecological generalists by virtue of a high
between-phenotype component of niche breadth (see also Roughgarden
1972). In contrast, species that are ecological specialists would be
expected to show low morphological variability and little variance
between individuals in resource utilization. Empirical support for
this morphological variation-niche width hypothesis has been slow in
coming; most attempts to test the hypothesis have relied on avian beak
measurements (Grant 1967,1971; Soule and Stewart 1970; Van Valen and
Grant 1970; Willson 1969).

Lanthanotus, the sister group to monitor lizards, from Borneo.
Size differences between closely-related sympatric species have been implicated as being necessary for coexistence (Hutchinson 1959; MacArthur 1972; Schoener 1965, 1983a), and even in the "assembly" of communities (Case et al 1983), although there has been considerable dispute over the statistical validity of these patterns (Grant 1972; Horn and May 1977; Strong et al 1979; Grant and Abbott 1980; Simberloff and Boecklen 1981).
Because anatomical parameters are usually much easier to estimate
objectively than ecological ones, a variety of recent studies attempt to exploit such morphological correlates of ecology to make anatomical maps of ecological space and, in turn, to use these to analyze various aspects of community structure; these efforts deal with vertebrate taxa as divergent as bats (Findley 1973, 1976), birds (Karr and James 1975; Ricklefs and Travis 1980), fish (Gatz 1979a, 1979b), and lizards (Ricklefs et al. 1981). [This study, based on my own data on lizard anatomy, is revised and extended here.] In this approach, each species is represented as a point in an n-dimensional hypervolume whose coordinates are the morphological variates. These may be standardized as desired or log transformed. Euclidean distances between species are calculated as measures of dissimilarity. Distances from the centroid of the hypervolume can be exploited to judge the overall size of
morphological space. If desired, dimensionality can be reduced and orthogonality achieved by a multivariate procedure such as principal components analysis (note that euclidean distances remain unchanged when axes are rotated). Spacing patterns between species, such as nearest neighbor distances, and other aspects of their position in morphological space can then be examined. The assumption is usually
made that the arrangement of species in morphological space accurately reflects their ecological relationships, although this assumption is not easily verifiable and has seldom been directly tested [but see Ricklefs and Travis (1981) and below].
For most individual lizards collected, ten morphometrics were measured: snout-vent and tail length, the length, width, and depth of the head, and the lengths of the jaw, forefoot, forearm, hindfoot, and hindleg. Even though sexual dimorphisms do occur in some species, sexes are lumped for simplicity and ease of analysis here. These morphometrics proved to be strongly positively correlated with one another over all 90-odd species (mean correlation coefficient = 0.75, standard deviation = 0.148). Anatomical statistics for 92 species are summarized in Appendix G in Pianka (1986). Average morphometrics were used to represent each species as a point in a multidimensional morphospace. Each morphometric was given equal weight by standardizing by subtraction of the mean value for all 92 species and division by the standard deviation across all species: this standardization procedure results in a mean score of 0.0 and a standard deviation of 1.0 for each variate. [In an earlier effort (Ricklefs et. al. 1981), morphometrics were log-transformed, which required omission of aberrant legless skinks and pygopodids. These species are included in the present analysis.] Distances from this standardized hypervolume's centroid (representing the overall "average" lizard species) were calculated for each species and averaged for each continental saurofauna. Euclidean distances between all pairs of species were computed, and nearest neighbor distances identified for each. Various morphometric statistics, computed for three large Australian genera and for each continent separately as well as for all intracontinental plus intercontinental pairs of species, are summarized in Table 11.2 in Pianka 1986.
Anatomically, Kalahari lizard species are appreciably more similar than are the North American or Australian lizard species. Both the overall average and nearest neighbor euclidean distances are smaller and less variable in Kalahari lizards than on the other two continental-desert lizard systems. Euclidean distances between species are most variable
in Australia, probably partially due to the larger number of species
there. The overall volume of morphospace occupied by Kalahari lizards,
as judged by distances from the centroid (Table 11.2 in Pianka 1986), is more compact
than that occupied by lizards in North America and Australia. To
reduce dimensionality, a principal components analysis of these data
was undertaken. When all 91 species are considered together, the first
three principal components reduce overall variance by 77.7%, 11.6%, and
6.7% (total 96%), respectively. Positions of various species on these
first three principal components of morphospace are depicted in Figures
11.4, 11.5, and 11.6. Centroids for each of the continental faunas deviate from the overall centroid of all 91 species (marked by the intersections of the origins of all three principal components on Figures 11.4 to 11.6 in Pianka 1986).

Texas horned lizard,
Phrynosoma cornutum.
Interestingly enough, even on these very crude
morphological dimensions, the Australian ant specialist Moloch horridus and its American "ecological equivalent," the horned lizard Phrynosoma platyrhinos, are actually closer to one another than either is to another member of its own saurofauna.
Finally, the best available estimates of overall ecological
similarity, the products of microhabitat times dietary overlap (from
merged deck analyses), were compared directly to these anatomical
distances. As expected, product moment correlation coefficients
between ecological similarity and morphological separation are
negative. Most such correlations are weak but still statistically
significant. The strongest of these inverse correlations,
is for Kalahari lizards (see Figure 11.7 in Pianka 1986). Although anatomically
similar species proved to be widely variable in the extent of their
ecological overlap, morphologically disparate species are inevitably
ecologically distinct.
The present data set can also be exploited to test the
morphological variation-niche width hypothesis in at least two
different ways. Estimates of microhabitat niche breadth (Appendix C in Pianka 1986)
and those for dietary niche breadth (Appendix E in Pianka 1986) can be compared
directly to appropriate measures of anatomical variability, namely the
coefficients of variation for hindleg length and head length (Table
11.4 in Pianka 1986). While somewhat equivocal, these tests do tend to support the
hypothesis that generalists are morphologically more variable than specialists.
References on Ecomorphology
Ananjeva, N. B. 1977. Morphometrical analysis of limb proportions of five sympatric species of desert lizards (Sauria, Eremias) in the southern Balkhash lake region. Proceedings of the Zoological Institute, Academy of Sciences of the U.S.S.R. 74: 3-13.
Case, T. J. 1979. Character displacement and coevolution in some Cnemidophorus lizards. Fortschr. ZooI. 25: 235-282.
Case, T. J., J. Faaborg, and R. Sidell. 1983. The role of body size in the assembly of West Indian bird communities. Evolution 37: 1062-1074.
Collette, B. B. 1961. Correlations between ecology and morphology in anoline lizards from Havana, Cuba and southern Florida. Bulletin, Museum of Comparative Zoology, Harvard University 125: 137-162.
Findley, J. S. 1973. Phenetic packing as a measure of faunal diversity. American Naturalist 107: 580-584.
Findley, J. S. 1976. The structure of bat communities. American Naturalist 110: 129-139.
Gatz, A. J. 1979a. Community organization in fishes as indicated by morphological features. Ecology 60: 711-718.
Gatz, A. J. 1979b. Ecological morphology of freshwater stream fishes. Tulane Studies in Zoology and Botany 21: 91-124.
Grant, P. R. 1967. Bill length variability in birds of the Tres Marias islands, Mexico. Canadian Journal of Zoology\& 45: 805-815.
Grant, P. R. 1971. Variation in tarsus length of birds in island and mainland regions. Evolution 25: 599-614.
Grant, P. R. 1972. Convergent and divergent character displacement. Biological J. Linnaean Society 4: 39-68.
Grant, P. R. and I. Abbott. 1980. Interspecific competition, island biogeography and null hypotheses. Evolution 34: 332-341.
Green, R. H. 1971. A multivariate statistical approach to the Hutchinsonian niche: bivalve molluscs of central Canada. Ecology 52: 543-556.
Hespenheide, H. A. 1973. Ecological inferences from morphological data. Annual Review of Ecology and Systematics 4: 213-229.
Hecht, M. K. 1952. Natural selection in the lizard genus Aristelliger. Evolution 6: 112-124.
Hotton, N. 1955. A survey of adaptive relationships of dentition
to diet in the North American Iguanidae. American Midland Naturalist 53: 88-114.
Karr, J. R. and F. C. James. 1975. Ecomorphological configurations and convergent evolution in species and communities. Pages 258-291 ^&in\& M. L. Cody and J. M. Diamond, editors, "Ecology and Evolution of Communities". Harvard University Press.
Horn, H. S. and R. M. May. 1977. Limits to similarity among coexisting competitors. Nature 270: 660-661.
Hutchinson, G. E. 1959. Homage to Santa Rosalia, or why are there so many kinds of animals? American Naturalist 93: 145-159.
Lundelius, E. L. 1957. Skeletal adaptations in two species of Sceloporus. Evolution 11: 65-83.
MacArthur, R. H. 1972. Geographical Ecology\&. Harper and Row, N.Y.
269 pp.
Pianka, E. R. 1986. Ecology and natural history of desert lizards. Analyses of the ecological niche and community structure. Princeton University Press, Princeton, N.J.
Pianka, E.R. and H.D. Pianka. 1976. Comparative ecology of twelve species of nocturnal lizards (Gekkonidae) in the Western Australian desert. Copeia 440 1976: 125-142.
Ricklefs, R. E. and J. Travis. 1980. A morphological approach to
the study of avian community organization. Auk 97: 321-338.
Ricklefs, R. E., D. Cochran, and E. R. Pianka. 1981. A morphologi
cal analysis of the structure of communities of lizards in desert habitats. Ecology 62: 1474-1483.
Schoener, T. W. 1965. The evolution of bill size differences among sympatric congeneric species of birds. Evolution 19: 189-213.
Simberloff, D. S. and W. Boecklen. 1981. Santa Rosalia reconsidered. Evolution 35: 1206-1228.
Soule, M. and B. R. Stewart. 1970. The "niche-variation" hypothesis: A test and alternatives. American Naturalist 104: 85-97.
Strong, D. R., L. A. Szyska, and D. S. Simberloff. 1979. Tests of community-wide character displacement against null hypotheses. Evolution 33: 897-913.
Roughgarden, J. 1972. Evolution of niche width. American Naturalist 106: 683-718
Van Valen, L. and P. R. Grant. 1970. Variation and niche width reexamined. American Naturalist 104: 589-590.
Van Valen, L. 1965. Morphological variation and the width of the ecological niche. American Naturalist 100: 377-389.
Werner, Y. 1969. Eye size in geckos of various ecological types (Reptilia
: Gekkonidae and Sphaerodactylidae). Israel J. Zoology 18: 291-316.
Willson, M. F. 1969. Avian niche size and morphological variation. American Naturalist 103: 531-542.
Winemiller, K. O. 1991. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecological Monographs 61: 343-365.
Back to Herpetology
Home Page
Last updated 20 March 1997
by Eric Pianka
|
|