 The Many Dimensions of a Lizard's Ecological Niche Eric R. Pianka Abstract Lizards have proven themselves to be almost ideal organisms for ecological studies. Because they are ectotherms, they are often abundant, making them relatively easy to locate, observe, and capture. By facilitating metabolic inactivity on both a daily and a seasonal basis, ectothermy may well confer lizards with an advantage over birds under conditions of low and unpredictable productivity such as in desert regions. Lizards exhibit a wide range of variation in many morphological, behavioral, physiological, and ecological phenomena. Some species are annuals, whereas others live for decades. Reproductive tactics are readily studied and quite varied. Some species, such as geckos and the iguanian Anolis, have invariate small clutch sizes. Other species are exceedingly prolific, laying large clutches of several dozen eggs. Viviparity has arisen repeatedly among different lineages, as have fringed toe lamellae. Both arboreal and terrestrial lizards occur among both nocturnal and diurnal species. Some species such as Basiliscus are highly aquatic as well. Lizards inhabit a broad range of habitats, including deserts, grasslands, chaparral, rock outcrops, deciduous forest and rainforest. Lizards exhibit a wide range of thermoregulatory tactics, ranging along a continuum from passive thermoconformers to active thermoregulators. The slope of the regression of body temperature plotted against ambient temperature is a useful index of the position of any given species on this thermoregulation spectrum. A physiological design constraint is suggested by comparative analysis of 82 species from 10 families. This thermoregulation axis can also be exploited as a convenient unidimensional surrogate to represent multidimensional spatial and temporal niche dimensions. Diets are quite varied among lizards, with some species being generalists and others specializing on only a narrow range of prey. Lizards have specialized on ants, termites, scorpions, other lizards, birds, mammals, and even on some plant foods. Some lizards are ambush hunters, catching prey by sitting and waiting for it to move past. Others are more active, foraging widely for their prey. Although it is energetically more expensive to forage widely and probably more hazardous in terms of attracting the attention of potential predators, foraging widely can be advantageous in increasing contacts with potential prey. Phylogenetic systematics offers an exciting new framework and perspective for elucidating evolutionary pathways, evolutionary constraints and understanding lizard ecology. Even though the lizard niche is multidimensional and complex, it may eventually prove to be possible to construct a "periodic table of lizard niches" in a space of moderately low dimensionality. However, much more data than are presently available will be required to achieve this goal.  Introduction The ecological niche is defined as the sum total of the adaptations of an organismic unit, or as all of the various ways in which a given organismic unit conforms to its particular environment (Pianka 1974). The niche concept has gradually become inextricably linked to the phenomenon of interspecific competition, and, in the U.S. it is increasingly becoming identified with patterns of resource utilization (Pianka 1981). Niche relationships among competing species are frequently visualized and modeled with bell-shaped resource utilization curves along a continuous resource gradient, such as prey size or height above ground. Emphasis on resource use is operationally tractable. Lizard niches are multidimensional, complex and elusive. At least five major dimensions which are not independent should be recognized (Table 1). Space, time, and food correspond to the place niche, the temporal activity niche and the trophic or dietary niche. Reproductive tactics and predator escape tactics must also be included. The spatial niche includes habitat and microhabitat. The temporal niche includes both seasonal and diurnal patterns of activity. Use of space and time are inextricably intertwined. Habitat and Microhabitat Lizards inhabit a broad range of habitats, including deserts, grasslands, chaparral, rock outcrops, deciduous forest and rainforest. Certain species of lizards are climbers, others subterranean, while still others are surface dwellers (although most lizards exploit burrows as retreats). Among the latter, some tend to be found in open areas whereas others frequent the edges of vegetation. Because such spatial and temporal differences limit the frequency of encounters between species as well as expose them to differing food resources, any potential effects of interspecific competition would tend to be ameliorated. Indeed, avoidance of competition is perhaps the most plausible basis for the evolution and maintenance, and thus the very existence, of such niche differences. (Other possibilities, such as physiological design constraints and predator avoidance tactics, need also be considered.) We can learn a lot from careful study of lizard ecology. In the North American deserts, zebra-tailed lizards (Callisaurus draconoides) are usually in the open sun when first sighted; in contrast, side-blotched lizards (Uta stansburiana) are most frequently found underneath shrubs. Other iguanid species, such as Sceloporus magister and Urosaurus graciosus, are almost always found in trees at some distance above ground. Urosaurus frequents smaller branches in the tree canopy where it captures most of its insect prey, whereas Sceloporus use tree trunks as perches from which they make forays to the ground to feed (such species perhaps should not be labelled "arboreal" or "semiarboreal," but rather should be called climbing ground feeders). Such climbing lizards exploit a distinctly different microhabitat than true ground-dwelling species which forage at considerably greater distances away from trees; hence any potential for competition for food should be reduced by this differential use of space. Microhabitat utilization frequencies for various species of lizards in each of the three continental desert systems were summarized by Pianka (1986). Overall frequencies of use of various microhabitat elements, along with total numbers of undisturbed lizards observed in each, are listed in Table 2. Considerable intercontinental variation in the incidence of use of different microhabitats is evident: for example, in the two southern hemisphere deserts, substantially more lizards are arboreal and subterranean. The diversity of microhabitats used by various species of North American desert lizards (also termed "microhabitat niche breadth") varies from 1.0 in the specialized night lizard, Xantusia vigilis, (found only in the fallen rubble underneath Joshua trees) to 3.87 in the much more generalized side-blotched lizard, Uta stansburiana (found in 11 of the 14 microhabitats exploited by American lizards -- Pianka, 1986). Among all 11 species of North American desert lizards, microhabitat niche breadth averages 2.19 (standard deviation = 1.0, N = 11). In the southern hemisphere deserts, subterranean lizards add a 15th microhabitat resource state. Among 22 species of Kalahari lizards, observed microhabitat niche breadths vary from 1.0 in the very specialized fossorial Typhlosaurus skinks to nearly 6.0 in two species of climbing skinks (genus Mabuya) and the terrestrial lacertid Ichnotropis squamulosa (mean = 3.36, st. dev. = 1.44, N = 22). Mean microhabitat niche breadth is significantly greater (t = -.2.41, df = 31, P < .025) in the Kalahari than in North America. In many of the 60 Australian species, microhabitat niche breadth is low (mean = 3.02, st. dev. = 1.38, N = 60, Pianka 1986), due partially to small sample sizes (Australian microhabitat niche breadths do not differ significantly from those in North America or in the Kalahari by t-tests). Figure 1 summarizes these data. However, numbers of lizards observed and their microhabitat niche breadths are uncorrelated ( r = 0.037) -- many uncommon species, such as Menetia greyi and Heteronotia binoei, are nevertheless relatively generalized.  Microhabitat niche breadths are, on average, broadest in the Kalahari, narrowest in North America, and intermediate in Australia. Estimates of the diversity of microhabitats actually used by the entire saurofaunas of each of the study areas are listed in Table 3. Note that microhabitat diversity is lowest in North America and most variable from site to site in the Kalahari. The diversity of microhabitats used by Australian desert lizards is high. Temporal Patterns of Activity Even a casual observer quickly notices that various species of desert lizards differ markedly in their times of activity. Some species are active early in the morning, but other species do not emerge until late morning or midday. Most geckos and pygopodids and some Australian skinks are nocturnal. Times of activity of most lizards are relatively consistent from day to day and change more or less regularly with the seasons. Many species of diurnal lizards exhibit a bimodal daily pattern of activity (early-late) during the warm summer months, but a single mid-day period of activity at cooler times of the year. Such seasonal shifts in time of activity facilitate thermoregulation by allowing the lizards to encounter a similar thermal environment at different times of year. Standardizing times of activity to "time since sunrise" (diurnal species) or "time since sunset" (nocturnal species) corrects for such seasonal shifts in activity times and allows comparisons among species and between communities. Sympatric species often differ in their activity patterns, with some emerging earlier than others. Such sequential replacements of lizard species during the day are illustrated for four species of North American desert lizards (Figure 2) and for five sympatric species of Australian Ctenotus skinks (Figure 3). Differences in time of activity may result in exposure to different prey resources. Some species, such as the iguanid Dipsosaurus dorsalis, the scincid Ctenotus leae, and the lacertid Nucras tessellata, may actually avoid predators by being active during the heat of mid day. Thermoregulation Thermoregulation has been the focus of attention in lizard ecology and natural history since the classic paper of Cowles and Bogert (1944). Animals that maintain relatively constant internal body temperatures are homeotherms, whereas those whose body temperatures vary widely from time to time, often approximating the temperature of their immediate environment, are called poikilotherms. A related pair of useful terms are sometimes confused with these two terms. An ectotherm obtains its heat from its external environment, whereas an endotherm produces most of its own heat internally by means of oxidative metabolism. All plants and the vast majority of animals are ectothermic; the only continuously endothermic animals are found among birds and mammals (but even many of these become ectothermic at times). Some poikilotherms including large lizards are at times at least partially endothermic. Many ectothermic lizards actually regulate their body temperatures fairly precisely during periods of activity by appropriate behavioural means, thus achieving homeothermy. An active desert lizard may have a body temperature just as high as that of a bird or mammal (the misleading layman's terms "warm-blooded" and "cold-blooded" should be abandoned). When averaged over a long enough period of time, heat gained by an organism must be exactly balanced by heat lost to its environment; otherwise the animal either warms up or cools off. Many different pathways of heat gains and heat losses exist. Balancing a heat budget requires very different adaptations under varying environmental conditions. At different times of day, ambient thermal conditions may change from being too cold to being too warm for a particular lizard's optimal performance. Lizards living in hot deserts must avoid overheating by being able to minimize heat loads and to dissipate heat efficiently; in contrast, those that live in colder places such as at high altitudes must be adept at acquiring and retaining heat. Environmental temperatures fluctuate in characteristic ways at different places over Earth's surface, both daily and seasonally. In the absence of a long-term warming or cooling trend, environmental temperatures at any given particular place remain roughly constant when averaged over an entire annual cycle. The range in temperature within a year is much greater at high latitudes than it is nearer the equator. A lizard can balance its annual heat budget by being entirely passive and simply allowing its temperature to mirror that of its environment. Such a passive thermoregulator is known as a thermoconformer. Of course, it is also an ectotherm. Another extreme is to maintain an absolutely constant body temperature by physiological and/or behavioral means, dissipating (or avoiding) excess bodily heat during warm periods but retaining (or gaining) heat during cooler periods. Such creatures that carefully regulate their internal temperatures are known as thermoregulators. Both endotherms and ectotherms regulate their body temperatures. There is a continuum between the two extremes of perfect conformity and perfect regulation. Regulation is never perfect. Because thermoregulation clearly has costs and risks as well as profits, an emerging conceptual framework envisions an optimal level of regulation that depends on the precise form of the constraints and interactions among costs and benefits arising from a particular ecological situation (Huey and Slatkin, 1976). Thermoregulation often involves both physiological and behavioral adjustments; as an example of the latter, consider a typical terrestrial diurnal desert lizard. During the early morning, when ambient temperatures are low, such a lizard locates itself in warmer microclimates of the environmental thermal mosaic (e.g., small depressions in the open or on tree trunks), basking in the sun with its body as perpendicular as possible to the sun's rays and thereby maximizing heat gained. With the daily march of temperature, ambient temperatures quickly rise and the lizard seeks cooler shadier microhabitats. Individuals of some species retreat into burrows as temperatures rise; others climb up off the ground into cooler air and orient themselves facing into the sun's rays, thereby reducing heat load. Many lizards change colors and their heat reflectance properties, becoming dark and heat absorbent at colder times of day but light and heat reflectant at hotter times. Such adjustments allow individual lizards to be active over a longer time interval than they could be if they conformed passively to ambient thermal conditions; no doubt they are also more effective competitors and better able to elude predators as a result of such thermoregulatory behaviors. Lizards constitute an extremely conspicuous element of the vertebrate faunas of most deserts, especially warmer ones. Indeed, the Australian mammalogist Finlayson (1943) referred to the vast interior deserts of Australia as "a land of lizards." Like other ectotherms, lizards obtain their bodily heat solely from the external environment, as opposed to endotherms such as birds and mammals which can produce their own heat internally by means of oxidative metabolism. Moreover, along with other ectotherms, lizards are low-energy animals. Bennett and Nagy (1977) underscore the great "economy of the saurian mode of life" by pointing out that one day's food supply for a small bird will last a lizard of the same body size for over a month. Ectothermy presumably has distinct advantages over endothermy under the harsh and unpredictable conditions that prevail in deserts. By means of this thermal tactic, lizards can conserve water and energy by becoming inactive during the heat of midday, during resource shortages, or whenever difficult physical conditions occur (such as during heat waves or droughts). Birds and mammals must weather out these inhospitable periods at a substantially higher metabolic cost. Ectothermy thus confers lizards with the ability to capitalize on scant and unpredictable food supplies and other resources; presumably this gives lizards a distinct competitive advantage over endotherms in many desert environments. Hot, arid regions typically support rich lizard faunas, whereas cooler forested areas support fewer lizard species and individuals. Lizards can enjoy the benefits of a high metabolic rate during relatively brief periods when conditions are appropriate for their activity and yet can still become inactive during adverse conditions. By facilitating metabolic inactivity on both a daily and a seasonal basis, ectothermy thus allows lizards to capitalize on unpredictable food supplies. Moreover, most endothermic diurnal birds and mammals must wait out the hot midday period at considerable metabolic cost, whereas lizards can effectively reduce temporal heterogeneity by retreating underground, becoming inactive, and lowering their metabolic rate during harsh periods (some desert rodents estivate when food and/or water is in short supply). Ectothermy probably contributes to the apparent relative success of lizards over birds and mammals in arid regions (Schall and Pianka 1978). Forests and grasslands are probably simply too shady and too cold for ectothermic lizards to be very successful because these animals rely on basking to reach body temperatures high enough for activity; birds and mammals, in contrast, do quite well in such areas due to their ability to maintain activity via endothermy. Students of thermoregulation have often noted an apparent upper thermal limit of about 40oC for most of the Earth's eukaryotic creatures (most plants, invertebrates, and vertebrates). This thermal "lid" has frequently been used as evidence for an extremely archaic and inflexible fundamental physiological process (perhaps some enzyme fundamental to all life processes, such as a dehydrogenase, denatures). Major exceptions are certain heat-tolerant bacteria and blue-green algae, inhabitants of hot springs and oceanic volcanic vents. These prokaryotic organisms may well have arisen before the origin of the heat-sensitive metabolic pathway that limits eukaryotes. An example of such a physiological design constraint involves the thermal relationships of vertebrates, spanning classes from reptiles to mammals (Pianka, 1985, 1986). Detailed consideration of behavioral thermoregulation in lizards enables a fairly accurate prediction of the active body temperatures of mammalian homeotherms. A provocative biological "constant" can be identified that suggests a substantial degree of physiological inertia. An intriguing hypothesis for the evolution of homeothermy was offered by Hamilton (1973), who suggested that homeothermy is a by-product of advantages gained from maintaining maximum body temperatures in the face of such an innate physiological ceiling. Ecologically optimal temperatures do not always coincide with physiological optima (Huey and Slatkin 1976). Not all homeotherms are endotherms; many ectotherms such as lizards have attained a substantial degree of homeothermy by means of behavioral thermoregulation. Typically, these organisms actively select thermally suitable microhabitats, orient their bodies (or parts thereof) to control heat exchange, and/or shuttle between sun and shade as necessary to maintain a more-or-less constant internal body temperature. Thermoregulation in lizards is not nearly as simple as it might appear to be at first glance, but rather encompasses a wide diversity of very different thermoregulatory tactics among species ranging from ectothermic poikilothermy to and including ectothermic homeothermy. Thermal relations of active lizards vary widely among species and are profoundly influenced by their spatial and temporal patterns of activity. Body temperatures of some diurnal heliothermic species average 38oC or higher, whereas those of nocturnal thigmothermic species are typically in the mid-twenties, closely paralleling ambient air temperatures. Variance in body temperature varies between species as does the relationship between body temperatures and air temperatures. For example, among North American lizards, two arboreal species (Urosaurus graciosus and Sceloporus magister) display narrower variances in body temperature than do terrestrial species. Presumably, arboreal habits often facilitate efficient, economic, and rather precise thermoregulation. Climbing lizards have only to shift position slightly to be in the sun or shade or on a warmer or cooler substrate, and normally do not move through a diverse thermal environment. Moreover, arboreal lizards need not expend energy making long runs as do most ground dwellers, and thus climbing species do not raise their body temperatures metabolically to as great an extent as do terrestrial lizards. Such differences in temporal patterns of activity, the use of space, and body temperature relationships are hardly independent. Rather, they complexly constrain one another, sometimes in intricate and obscure ways. For example, thermal conditions associated with particular microhabitats change in characteristic ways in time; a choice basking site at one time of day becomes an inhospitable hot spot at a later time. Perches of arboreal lizards receive full sun early and late in the day when ambient air temperatures tend to be low and basking is therefore desirable, but these same tree trunks are shady and cool during the heat of midday when heat-avoidance behavior becomes necessary (Huey and Pianka 1977a). In contrast, the fraction of the ground's surface in the sun is low when shadows are long early and late, but reaches a maximum at midday. Terrestrial heliothermic lizards may thus experience a shortage of suitable basking sites early and late in the day; moreover, during the heat of the day, their movements through relatively extensive patches of open sun can be severely curtailed. Hence, ground-dwelling lizards encounter fundamentally different and more difficult thermal challenges than do climbing species. Radiation and conduction are the most important means of heat exchange for the majority of diurnal lizards, although the thermal background in which these processes occur is strongly influenced by prevailing air temperatures. Ambient air temperatures are critical to nocturnal lizards as well as to certain cryptic diurnal species. In an analysis of the costs and benefits of lizard thermoregulatory strategies, Huey and Slatkin (1976) identified the slope of the regression of body temperature against ambient environmental temperature as a useful inverse measure of the degree of passivity in regulation of body temperature. On such a plot of active body temperature versus ambient temperature, a slope of zero reflects the one extreme of perfect thermoregulation, whereas a slope of one indicates the other extreme of true poikilothermy or totally passive thermoconformity (air temperature and body temperature are perfectly correlated). Lizards span this entire thermoregulation spectrum. Among active diurnal heliothermic species, regressions of body temperature on air temperature are fairly flat (for several species, including some quite small ones, slopes do not differ significantly from zero); among nocturnal species, however, slopes of similar plots are typically closer to unity. Various other species (nocturnal, diurnal, and crepuscular), particularly Australian ones, are intermediate, filling in the continuum of thermoregulatory tactics. 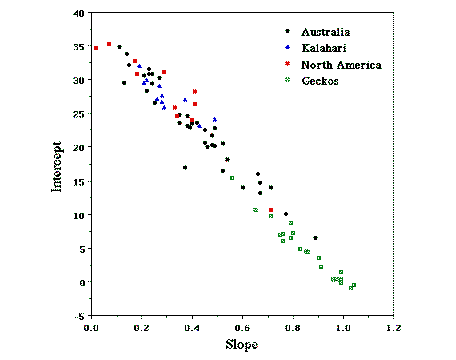 A straight line can be represented as a single point in the coordinates of slope versus intercept; these two parameters were plotted for linear regressions of body temperatures on air temperatures among some 82 species of lizards by Pianka (1986). Each data point represents the least-squares linear regression of body temperature against air temperature for a given species of desert lizard. These data points fall on yet another, transcendent, straight line (Figure 4). The position of any particular species along this spectrum reflects a great deal about its complex activities in space and time. The line plotted in this figure is thus a unidimensional surrogate for multidimensional spatial-temporal niche dimensions: it offers a potent linear dimension on which various species can be placed in attempts to formulate general schemes of lizard ecology (Pianka, 1985, 1986 -- see also last section of this paper). Various other ecological parameters, including reproductive tactics, can be mapped on to this emergent spatial-temporal axis. The intriguing "intercept" of the intercepts (38.8oC) approximates the point of intersection of all 82 regression lines and represents an innate design constraint imposed by lizard physiology and metabolism. It is no accident that this value also corresponds closely to the body temperatures of homeotherms, particularly mammals! Birds maintain slightly higher body temperatures than mammals (Hamilton, 1973), and descended from another reptilian stock, the archosaurs, represented today by the crocodilians. Would a comparable study of crocodilian thermoregulation yield a higher intercept of the intercepts? (This prediction could be doomed to failure by the mere fact that crocodilians are aquatic and very large -- yet they clearly thermoregulate when out of the water.) Although most insects are so small that convective heat exchange prevents them from attaining body temperatures much higher than that of ambient air, some, such as bumblebees and butterflies, do exhibit behavioral thermoregulation; would a plot for insects show more scatter and a different intercept? To continue reading this document, click The many dimensions of a lizard's ecological niche, continued. Books by Eric R. Pianka 
Return to Pianka lab page |