 Diet Certain species of lizards are dietary specialists, eating a narrow range of prey types. For example, the Kalahari lacertid Nucras tessellata and the Australian pygopodid Pygopus nigriceps both consume an excessive number of scorpions when compared to other lizard species (Pianka 1986). Nucras forages widely to capture these large arachnids by day in their diurnal retreats, whereas the nocturnal Pygopus sits and waits for scorpions at night above ground during the latter's normal period of activity. In North America, no desert lizard has become a scorpion specialist, but the small sand-swimming snake Chionactis occipitalis seems to have usurped this ecological role. Scorpions are solitary prey items, but they are extremely large and nutritious, facilitating evolution of dietary specialization. For similar reasons, specialization on other lizards as food items has evolved in North American leopard lizards (Crotaphytus wislizeni) as well as among most Australian varanids. Other lizard species have much more catholic diets, eating a considerably wider variety of foods. Dietary niche breadth also varies within species from time to time and from place to place as the composition of diets change with opportunistic feeding in response to fluctuating prey abundances and availabilities (Pianka 1986). However, the consistency of lizard diets is remarkable, suggesting a profound impact of microhabitat utilization, foraging mode, as well as various anatomical and behavioral constraints imposed by phylogeny.  Diets of nearly a hundred species of desert lizards were summarized by Pianka (1986) as percentages by volume of various foods in the overall diets of all specimens of each species on all study areas within each desert-lizard system. Close scrutiny of these data matrices reveals that some species specialize on scorpions, ants, termites, vertebrates, and/or on plants, whereas other species on each continent are much more generalized, eating an extremely wide variety of food categories. No centipedes were found in stomachs of North American lizards, and solpugids are not present in Australia. Food niche breadths* range from 1.06 to 6.53 (mean 4.07, st. dev. = 1.93, N = 11) among the 11 species of North American lizards, from 1.07 to 8.22 (mean 3.85, st. dev. = 2.09, N = 21) among 21 Kalahari species, and from 1.00 to 10.9 (mean 3.86, st. dev. = 2.28, N = 59) among the 60 Australian species (Figure 5). None of these intercontinental variations in food niche breadths are significantly different by t-tests. Estimates of food niche breadths are uncorrelated with the number of lizards on which they are based ( r = 0.11), providing evidence that sample sizes are adequate to characterize patterns of food utilization even among the rarer species. Indeed, species with broad diets are often, though not always, relatively uncommon. Across species, dietary niche breadth is weakly, but significantly (r = .27, P < .02), positively correlated with microhabitat niche breadth, an indication that food specialists tend to be restricted to fewer microhabitats than food generalists. 
Biologically significant variation between species in utilization of certain relatively minor food categories is evident: for example, in the diets of climbing lizard species, hemiptera-homoptera and mantids-phasmids as well as various flying insects (wasps, Diptera, and Lepidoptera) tend to be better represented than they are among terrestrial species. Likewise, geckos consume more nocturnal arthropods (scorpions, crickets, roaches, and moths) than do most diurnal species (some diurnal lizards such as Nucras do capture nocturnal prey in their diurnal retreats). Such prey items are thus indicators of spatial and temporal patterns of activity. Overall diets of entire saurofaunas are summarized and compared in Table 4. Relatively few foods dominate lizard diets. Prey resource spectra are broadly similar between continents (Pianka 1986), although notable differences occur. In North America, the seven most important (totalling 84%), in decreasing order by volumetric importance, are: beetles, termites, insect larvae, grasshoppers plus crickets, ants, plant materials, and vertebrates. In the Kalahari, just three food categories far outweigh all others (total 71%): these are termites, beetles, and ants. In Australia, the five most important categories (total 77%, in decreasing order) are: vertebrates, termites, ants, grasshoppers plus crickets, and beetles. The same three categories, termites, beetles and ants, constitute major prey items in all three continental desert-lizard systems. Termites assume a disproportionate role in the Kalahari, as do vertebrate foods in Australia (largely a reflection of varanid diets). Somewhat surprisingly, the overall diversity of foods consumed by all species of lizards is actually greatest in the least diverse North American saurofauna (8.7), lowest in the Kalahari lizards (4.4), and intermediate in Australia (6.6). Basically comparable figures, although broadly overlapping, emerge from an area-by-area analysis (Pianka 1986, 1989). Prey diversity is weakly correlated with certain measures of the variability in average annual precipitation: food diversity is positively correlated with the coefficient of variation in annual precipitation (r = .45, P < .05), but is negatively correlated with the mean minus the standard deviation in precipitation. More variable precipitation, and presumably primary productivity, fosters higher insect species diversities (lizard diversity also correlates with variability of precipitation). Modes of Foraging Some predators attack their prey from ambush, whereas others usually hunt while on the move. Over 30 years ago, I termed these two modes of foraging the "sit-and-wait" versus the "widely-foraging" tactic, respectively (Pianka 1966). Of course, this dichotomy could be somewhat artificial, although numerous animal groups seem to fall rather naturally into either one category or the other. Members of most lizard families typically exploit either one or the other of these two modes of foraging: thus members of the iguanian lineage primarily sit and wait for their prey, whereas scleroglossans (teids, skinks and varanids) forage widely. Lacertids, however, exploit both modes of foraging, even within the same genus. This evidently natural dichotomy in foraging tactics has had a substantial impact on theories of optimal diets and competitive relationships among species (Magnusson et al. 1985; McLaughlin 1989; Perry et al. 1990; Pietruszka 1986). Empirical study of the influence of mode of foraging on diets has lagged behind theory. (One of my ex graduate students, Gad Perry, is working to close this gap.) Certain dietary differences are associated with this apparent dichotomy in foraging tactics. Sit-and-wait predators rely largely on moving prey whereas widely-foraging predators encounter and consume non-moving types of prey items more frequently. For the sit-and-wait tactic to pay off, prey must be relatively mobile and prey density must be high (or predator energy requirements low). The sit-and-wait tactic should be less prevalent during periods of prey scarcity than the widely-foraging method. The success of the widely-foraging tactic is also influenced by prey mobility and prey density as well as by the predator's energetic requirements (which should usually be higher than those of sit-and-wait predators), but the searching abilities of the predator and the spatial distribution of its prey now assume substantial importance. North American and Australian sites support similar numbers of species of sit-and-wait foragers, whereas this mode of foraging is distinctly impoverished in the Kalahari (Pianka 1986). Markedly fewer species forage widely in western North America (only one species, the teiid Cnemidophorus tigris) and in the Kalahari (an average of 4 species per site) than in the Australian deserts (mean number of widely-foraging species per area is 10.1, most of which are skinks in the genus Ctenotus). Intercontinental comparisons of proportions of total species in various foraging modes are also instructive: a full 60% of North American lizard species are sit-and-wait foragers, compared to only 16% in the Kalahari and 18% in Australia; percentages of widely-foraging species are 14% (North America), 27% (Kalahari), and 36% (Australia).  Two species of Kalahari lizards, Pedioplanis lineoocellata and Meroles suborbitalis, sit-and-wait for prey, whereas two other syntopic species, Heliobolus lugubris and Pedioplanis namaquensis), forage widely for their food (Pianka et al. 1979; Huey and Pianka 1981). Time budgets of these lacertids reflect their modes of foraging (Pianka 1986). Foraging widely is energetically expensive and, judging from their relative stomach volumes, those species that engage in this mode of food gathering appear to capture more prey per unit time than do sit-and-wait species. Overall energy budgets of widely-foraging species are nearly twice as great as those of sit-and-wait species (Huey and Pianka 1981). Sedentary foragers tend to encounter and eat fairly mobile prey whereas more active widely-foraging predators consume less active prey. Compared with sit-and-wait species, widely-foraging lacertid species eat more termites (sedentary, spatially and temporally unpredictable but clumped prey).  One widely-foraging species, Nucras tessellata, specializes on scorpions (by day, these large arachnids are non-mobile and exceedingly patchily-distributed prey items). Another ramification of foraging mode in these Kalahari lizards concerns exposure to their own predators. Because of their more-or-less continual movements, widely-foraging species expose themselves and tend to be more visible. As a result, they seem to suffer higher predation rates. Widely-foraging species fall prey to lizard predators that hunt by ambush whereas sit-and-wait lizard species tend to be eaten by predators that forage widely, generating "crossovers" in foraging mode between trophic levels. Widely-foraging lizard species are also more streamlined and have longer tails than sit-and-wait species (Huey and Pianka 1981).
One widely-foraging species, Nucras tessellata, specializes on scorpions (by day, these large arachnids are non-mobile and exceedingly patchily-distributed prey items). Another ramification of foraging mode in these Kalahari lizards concerns exposure to their own predators. Because of their more-or-less continual movements, widely-foraging species expose themselves and tend to be more visible. As a result, they seem to suffer higher predation rates. Widely-foraging species fall prey to lizard predators that hunt by ambush whereas sit-and-wait lizard species tend to be eaten by predators that forage widely, generating "crossovers" in foraging mode between trophic levels. Widely-foraging lizard species are also more streamlined and have longer tails than sit-and-wait species (Huey and Pianka 1981).Kalahari desert lacertid Nucras tessellata. Another important spin-off of foraging mode involves reproductive tactics. Clutch sizes of widely-foraging species are smaller than those of sit-and-wait species, probably because the former simply cannot afford to weight themselves down with eggs to as great an extent as can the latter (Vitt and Congdon 1978). Hence foraging style constrains reproductive prospects (as well as vice versa). Huey and Pianka (1981) summarize many of these ecological correlates of foraging mode. In an environment with a scant food supply, a consumer presumably cannot afford to bypass many inferior prey items because mean search time per item encountered is long and expectation of prey encounter is low (MacArthur and Pianka 1966). In such an environment, a broad diet maximizes returns per unit expenditure, favoring generalization. In a food-rich environment, however, search time per item is low because a foraging animal encounters numerous potential prey items. Under such circumstances, substandard prey items can be bypassed economically because expectation of finding a superior item in the near future is high. Hence rich food supplies favor selective foraging and lead to narrow food niche breadths. These arguments are supported by the North American teiid lizard Cnemidophorus tigris, which eats a greater diversity of foods in drier than average years (presumably times of low food availability) but like most lizards contracts its diet during periods of prey abundance (Pianka 1986). Another, more extreme, example of this phenomenon occurs after heavy summer rains when termites send out their winged reproductives in great abundance and virtually every species of lizard eats nothing but termites (even lizard species that normally never consume termites). During such fleeting moments of extraordinary prey abundance, competition for food is negligible and dietary overlap among members of a desert saurofauna is sometimes nearly complete. Reproductive Tactics Most lizards lay eggs, but some species retain their eggs internally and give birth to living young. Viviparity has arisen at least 25 times among lizards (Shine and Bull 1985) in seven different families (agamids, anguids, chameleons, geckos, phrynosomatids, lacertids, and skinks). Clutch or litter sizes vary from one to forty or more among different species of lizards. Some species reproduce only once every second or third year, others but once each year, while still others lay two or more clutches each year. Substantial spatial and temporal variation in clutch size also exists within species. As just one among many possible examples, in the double-clutched Australian agamid species Ctenophorus isolepis, the size of 67 first clutches (August-December) averaged 3.01 eggs whereas the mean of 41 second clutches (January-February) was 3.88 (Pianka 1971). Females grow during the season, and, as in many lizards, larger females tend to lay larger clutches. Interestingly enough, however, these same females invest relatively more on their second clutches than they did on their first clutch: among 25 first clutches, clutch volumes average only 11.2% of female weight, but in 15 second clutches the average was 15.1%. (95% confidence intervals on these means are non-overlapping -- 10.25 to 12.20 versus 13.38 to 16.85, respectively). Changes in fecundity with fluctuations in food supplies and local conditions from year to year or spot to spot have also frequently been observed: for example, in the North American whiptail Cnemidophorus tigris (Family Teiidae) females lay larger clutches in years with above-average precipitation and presumably ample food supplies (Pianka 1970, 1986). Similar phenomena have also been documented in Xantusia vigilis (Zweifel and Lowe 1966) and Uta stansburiana and doubtlessly occur in many or even most other lizard species. Clutch or litter weight (or volume), expressed as a fraction of a female's total body weight (known as "relative clutch mass" or RCM), ranges from as little as 4 to 5% in some species to as much as 20-30% in others. Clutch weights tend to be particularly high in some of the North American horned lizards (genus Phrynosoma). Ratios of clutch or litter weight to female body weight correlate strongly with various energetic measures and have often been used as crude indices of a female's instantaneous investment in current reproduction (sometimes equated with the elusive notion of "reproductive effort"). In addition to clutch size and female total investment in reproduction, the size (or weight) of an individual oviductal egg or newborn progeny, also varies widely among lizards from as little as 1-2% in some species to a full 17% in the live-bearing Kalahari fossorial skink Typhlosaurus gariepensis. Such expenditures per progeny are inverse measures of the extent to which a juvenile lizard must grow to reach adulthood. Of course, any two parties to this triad (clutch size, female reproductive investment, and expenditure per progeny) uniquely determine the third: however, forces of natural selection molding each differ substantially. Thus clutch or litter weight presumably reflects an adult female's best current investment tactic in a given environment at a particular instant in time whereas expenditure on any given individual progeny is probably more closely attuned to the average environment to be encountered by a juvenile. In a sense, then, clutch (or litter) size is the direct result of the interaction between an optimal parental reproductive tactic and an optimal juvenile body size (clutch size is, of course, simply the ratio of the former divided by the latter). Statistics on clutch/litter sizes, total reproductive investment of females, and expenditure per progeny among 65 species of desert lizards were presented by Pianka (1986), along with similar data on another 20 species of lizards, including both desert and non-desert forms extracted from the literature. Among the species surveyed, average clutch/litter size varies from 1 in the Kalahari skink Typhlosaurus gariepensis and the geckos Gehyra variegata and Ptenopus garrulus to 13 in the Kalahari agamid Agama hispida. Clutch sizes in certain horned lizards are still larger, averaging 24.3 in the American iguanid Phrynosoma cornutum (the Texas horned lizard). Clutch or litter size and female investment are significantly positively correlated (r = .482, P < .001), although scatter is considerable. Viviparous species (mostly skinks) tend to have slightly higher investment ratios than do the egg layers. Since expenditure per progeny can be estimated from total investment divided by clutch/litter size, it tends to decrease exponentially with increasing number of progeny (for a fixed total investment). Expenditure per progeny and clutch/litter size are inversely related (r = -.652 P < .001). This correlation is strengthened when both variables are transformed to logarithms (on a log-log plot, the correlation coefficient is -.810). Clutch size is thus correlated positively with total investment but negatively with investment per progeny. In simple product-moment correlation, the latter two members of the triumvirate, total reproductive investment and expenditure per progeny, are only weakly and not significantly correlated (r = .153, P < .10), suggesting that these two parameters vary independently of one another and that they may be responsive to different selective pressures. However, when effects of clutch size are held constant by partial correlation, the weak correlation between reproductive effort and expenditure per progeny is substantially improved (partial correlation coefficient = .704), an indication, once again, that these three aspects of reproductive tactics are far from independent of one another. Indeed, pairwise partial correlation coefficients between the logarithms of these three variates are all nearly perfect (rxy.z's = .910, .924, and -.969). Species fall neatly on a plane in this three space, as evidenced by a principal component analysis using log-transformed variables, which shows that the first two principal components reduce variance by over 99% (Pianka 1986). Frequency distributions of average clutch/litter sizes, total investment ratios, and expenditure per progeny among species were summarized by Pianka (1986). Expenditure per progeny varies over more than an order of magnitude, from 1% to 17% of a gravid female's body weight. Interestingly, species with narrow diets often though not always tend to have higher than average expenditures per progeny. Two of the species with the highest expenditures per progeny, Typhlosaurus gariepensis and T. lineatus, probably experience intense competition: (1) these live-bearing, subterranean skinks exist at very high population densities, (2) individuals are long-lived with delayed maturity, (3) litter sizes are extremely small (means of 1.0 and 1.5, respectively), and (4) females very likely reproduce only biennially (Huey et al. 1974). These two Kalahari fossorial skinks are also extreme food specialists, eating termites to the virtual exclusion of all other prey. The extremely high expenditure per progeny of Typhlosaurus may well be necessary to confer newborn animals with competitive ability sufficient to establish themselves in the highly competitive underground environment. Limited evidence indicates that investment per progeny is indeed responsive to and indicative of a lizard's competitive environment. Thus, in Typhlosaurus lineatus, offspring are significantly heavier (and expenditure per progeny significantly greater) where this species occurs in sympatry with T. gariepensis as compared with allopatric populations (Huey et al. 1974). Other food-specialized species seem also to encounter intense competition: among Australian geckos, species with relatively restricted termite diets tend to lay comparatively larger eggs and hence have higher expenditures per progeny than do those with more catholic diets (Pianka and Pianka 1976). A similar phenomenon appears to occur in the semi-arboreal African skink Mabuya spilogaster: on one study area, it is syntopic with an ecologically very similar species, Mabuya striata. Expenditure per progeny in M. spilogaster increases significantly (t-test, P < .01) from allopatry (mean = 4.39 + 0.21, N = 51) to sympatry (mean = 5.63 + 0.48, N = 19). Differences between viviparous and oviparous species are relatively slight, although, as noted above, viviparous species appear to invest slightly more in reproduction. Statistically significant differences exist between diurnal and nocturnal species of lizards in these reproductive statistics: nocturnal species have significantly smaller clutch/litter sizes and lower total investment in reproduction, but significantly higher expenditure per progeny (Pianka 1986). These differences between diurnal and nocturnal species stem largely from a simple historical or phylogenetic basis, since geckos and pygopodids dominate the nocturnal saurofauna and have a fixed clutch of only one or two eggs. Two viviparous nocturnal skink species (genus Egernia) also tend to have small litters; however, a third oviparous nocturnal skink, Sphenomorphus richardsoni, does not have a small clutch size. Reproductive tactics can be mapped, to a limited extent, on to the spatial-temporal thermoregulation axis plotted earlier. Simple pair-wise product-moment correlation coefficients between the three reproductive variables and the slope-intercepts of body temperature regressions on air temperature are weak although generally statistically significant. The strongest correlation, between the logarithm of clutch size and the intercept of body temperature on air temperature (r = .609), seems to arise largely as a result of the small clutch sizes and low body temperature intercepts of nocturnal lizards. Dunham and Miles (1985) undertook a detailed multivariate analysis of the reproductive tactics of 91 species of lizards. A discriminant function analysis describes an axis which has actively foraging species with large body sizes and small clutches at one end and sit-and-wait foragers with small body size and large clutches at the other end. This axis could be exploited as a dimension for construction of a "periodic table of lizard niches" (see last section). Predator Escape Tactics Lizard tails have diversified greatly and serve a wide variety of functions for their possessors. Many climbing species, such as the Australian sandridge agamid Gemmatophora longirostris, have evolved extraordinarily long tails which serve as effective counterbalances. Long tails enable lizards to raise their forelegs up off the ground and to run on their hind legs alone (bipedality is a faster means of locomotion than tetrapodality). Prehensile tails are used as a fifth leg in climbing by some arboreal lizard species like some geckos (e.g., Diplodactylus elderi) and by the true chameleons (Chameleo). In several members of the Australian gekkonid genus Diplodactylus (D. ciliaris, D. elderi, D. strophurus and relatives), glandular tails secrete and store a smelly noxious mucous. When disturbed, these lizards squirt out large amounts of sticky odoriferous gorp. Surprisingly, tails of these geckos are fragile and easily shed (but quickly regenerated). A related Australian desert gecko Diplodactylus conspicillatus has a non-glandular but very short and stubby bony tail: these nocturnal termite specialists hide in the vertical shafts of abandoned spider holes during the day and it is thought that they point head downwards and use their tails to block off these tunnels. Another Australian desert lizard with a similar yet different tail tactic is the climbing skink Egernia depressa. These lizards wedge themselves into tight crevices in mulga tree hollows (and rocks), blocking off the entrance with their strong and very spiny tails. Spinily-armored tails are used by numerous other species of lizards in a similar fashion, including the Mexican iguanid Enyaliosaurus clarki and the Saharan agamid Uromastix acanthinurus. 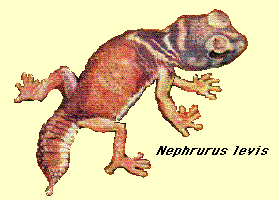 Members of another bizarre group of Australian lizards (genus Nephrurus) possess a unique round knob at the tip of their tails. These large nocturnal lizards eat big prey including other species of geckos on occasion. Both sexes carry the curious knob, but its function remains a total mystery. Unlike most geckos, their tails are not exceedingly fragile. In many species of lizards (especially among juveniles), tails are brightly colored and/or very conspicuous, evidently functioning as a lure to attract a potential predator's attack away from the more vulnerable and less dispensible parts of the animal. When approached or followed by a large animal, the zebra-tailed lizard of the western North American deserts, Callisaurus draconoides, curls its tail up over its hindquarters and back, exposing the bold black and white pattern underneath and coyly wriggling its tail from side to side. If pursued farther, zebra-tailed lizards resort to extreme speed (estimated at up to 20-30 km./hr.) and long zig-zag runs. An Australian desert skink, Ctenotus calurus, lashes and quivers its bright azure blue tail alongside its body continuously as it forages slowly through the open spaces between plants. Similarly, tiny Morethia butleri juveniles twitch their bright red tails as they move around in the litter beneath Eucalyptus trees. Tails of many, but by no means all, lizards break off easily. Indeed, some species can actually lose their tails voluntarily with minimal external force in a process known as autotomy (Arnold 1988). Freshly dismembered tails or pieces thereof typically thrash around wildly, presumably attracting a predator's attention while the recent owner quietly slips away unnoticed (Vitt et al. 1977). Some skinks, including many Ctenotus, return to the site where their tail was lost and swallow the remains of their own tail! Few, if any, other vertebrates display auto-amputation and self cannibalism. Many such lizards possess special adaptations for tail loss, including weak fracture planes within each tail vertebra, muscular attachments that facilitate autotomy and tail movement after dismemberment, as well as mechanisms for rapidly closing off blood vessels and healing. Losing its tail has surprisingly little effect on a lizard, as individuals often resume basking and foraging as if nothing had happened within minutes. In such lizard species, of course, tails are quickly regenerated from the stub. Although regrown tails are occasionally almost indistinguishable from the original externally, their internal support structure is cartilaginous rather than bony. Not all lizard tails are easily broken, however. Whereas most iguanids have fragile tails, their close relatives the agamids generally do not. Tails of varanids and true chameleons do not break easily either. Lizards with such tough tails usually cannot regenerate a very complete tail if their original should happen to be lost. The evolutionary bases for these differences, sometimes between fairly closely-related groups of lizards, are evasive. Arnold (1988) argues that easy tail loss is a primitive trait among lizards which has been lost but regained repeatedly among various lizard lineages. Certain small predators, such as the pygmy varanids Varanus gilleni and V. caudolineatus, may actually "harvest" the exceedingly fragile tails of geckos that are too large to subdue intact (Pianka 1969). Tail break frequency could serve as an index to the intensity of predation on lizard populations. It has since been used to attempt to estimate the amount of predation, although there are serious problems and limitations with the procedure (Schoener 1979). Efficient predators that leave no surviving prey obviously will not produce broken tails, but nevertheless may exert substantial predation pressures: broken and regenerated tails may therefore reflect lizard escape ability or predator inefficiency better than intensity of predation. Predator densities increase from north to south in western North America (Pianka 1986; Schall and Pianka 1980). Correlated with this latitudinal increase in predation, frequencies of broken and regenerated tails are higher at southern sites than at northern localities among four of the five widely-distributed lizard species. In the well-studied species Cnemidophorus tigris, frequency of broken tails decreases with latitude; moreover, diversity of predator escape behaviors utilized among members of these various Cnemidophorus populations also increases with the frequency of broken and regenerated tails (Schall and Pianka 1980). A greater variety of escape tactics, a form of behavioral "aspect diversity" (Rand 1967), presumably reduces the ease with which predators can capture lizard prey. In the Kalahari desert of southern Africa, juvenile lacertid lizards of the species Heliobolus lugubris employ an interesting anti-predator tactic involving deception known as Batesian mimicry (Huey and Pianka 1977b). These defenseless small lizards mimic noxious "Oogpister" beetles (the Afrikaans translates euphemistically as "eye squirter"), which emit pungent acids, aldehydes, and other chemicals when disturbed. Adult H. lugubris lizards are buff-colored and pale red, matching the color of Kalahari sands. Bodies of juveniles are jet black with white spots (juvenile tails are red matching the sand color). Whereas adults walk with a normal tetrapod lizard gait, with their backs undulating from side to side, juveniles walk stiff-legged, with backs arched vertically holding their reddish tails flat against the ground (this makes the tail difficult to detect). When pursued, young H. lugubris abandon their "beetle walk" and dart rapidly for cover, using normal lizard locomotion. As they reach a size of about 45-50 mm from snout to vent (the size of the largest oogpister beetles), these lizards "metamorphose" into the cryptic adult coloration and permanently abandon the stilt walk. The frequency of broken and regenerated tails is lower in juvenile H. lugubris than among closely related lacertids in the same habitats exposed to common predators, suggesting that this beetle mimicry does reduce predatory attacks. To continue reading this document, click The many dimensions of a lizard's ecological niche, continued. Books by Eric R. Pianka 
Return to Pianka lab page |