 Fringed Toes and Shovel Noses An open sandy desert poses severe problems for its inhabitants: (1) windblown sands are loose and provide little traction; (2) surface temperatures at midday rise to lethal levels; and (3) open sandy areas offer little food or shade or cover for evading predators. Even so, natural selection over eons of time has allowed lizards to cope fairly well with such sandy desert conditions. Subterranean lizards simply bypass most of the problems by staying underground, and actually benefit from the loose sand since underground locomotion is made easier. Burrowing is also facilitated by the evolution of a pointed, shovel-shaped head and a countersunk lower jaw, as well as by small appendages and muscular bodies and tails.  During the hours shortly after sunrise, but before sand temperatures climb too high, diurnal lizards scurry about above ground in such sandy desert habitats. Sand specialized lizards provide one of the most striking examples of convergent evolution and ecological equivalence. Representatives of many different families of lizards scattered throughout the world's deserts have found a similar solution for getting better traction on loose sand: enlarged scales on the toes, or lamellae, have evolved independently in five different families of lizards: skinks, lacertids, phrynosomatids, agamids, and geckos (Luke 1986). A skink, appropriately dubbed the "sand fish," literally swims through sandy seas in search of insect food in the Sahara and other eastern deserts. These sandy desert regions also support lacertid lizards (Acanthodactylus) with fringed toes and shovel noses. Far away in the southern hemisphere, on the windblown dunes of the Namib desert of southwestern Africa, an independent lineage of lacertids has evolved a similar life form, Aporosaura anchietae, illustrated above. In North America, this body form has been adopted by members of the iguanid genus Uma, which usually forage by waiting in the open and eat a fairly diverse diet of various insects, such as sand roaches, beetle larvae and other burrowing arthropods. They also listen intently for insects moving buried in the sand, and dig them up. Sometimes they dash, dig, and paw through a patch of sand and then watch the disturbed area for movements. All of these lizards have flattened, duckbill-like, shovel-nosed snouts, which enable them to make remarkable "dives" into the sand even while running at full speed. The lizards then wriggle along under the surface, sometimes for over a meter. One must see such a sand diving act to appreciate fully its effectiveness as a disappearing act. Some Namib desert lizards discovered another solution to gain traction on powdery sands: frog-like webbing between the toes as seen in the gecko Palmatogecko.  Interdependence of Niche Dimensions Any given organism possesses a unique coadapted complex of physiological, behavioral, and ecological traits whose functions complement one another and enhance that organism's reproductive success. Such a constellation of adaptations has been called an optimal design (Rosen, 1967) or an adaptive suite (Bartholomew, 1972). 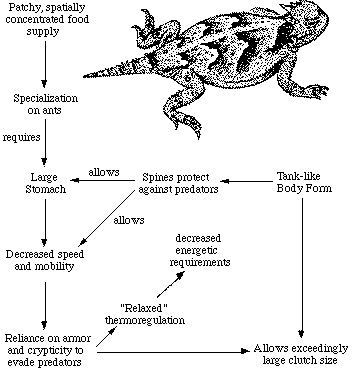 The adaptive suite of anatomical, behavioral, and ecological factors influencing an ant specialist, the desert horned lizard Phrynosoma platyrhinos. Consider the desert horned lizard Phrynosoma platyrhinos (Figure 6). Various features of its anatomy, behavior, diet, temporal pattern of activity, thermoregulation, reproductive tactics, and predator escape tactics, can be profitably interrelated and interpreted to provide an integrated view of the ecology of this interesting animal (Pianka 1966; Pianka and Parker, 1975). Horned lizards are ant specialists and usually eat essentially nothing else. Ants are small and contain much undigestible chitin, so that large numbers of them must be consumed. An ant specialist must therefore possess a large stomach for its body size. When expressed as a proportion of total body weight, the stomach of this horned lizard occupies a considerably larger fraction of the animal's overall body mass (about 13 percent) than do the stomachs of all other sympatric desert lizard species, including the herbivorous desert iguana Dipsosaurus dorsalis (herbivores typically have lower assimilation rates and larger stomachs than carnivores). Possession of such a large gut necessitates a tanklike body form, reducing speed and decreasing the lizard's ability to escape from predators by flight. As a result, natural selection has favored a spiny body form and cryptic behavior rather than a sleek body and rapid movement to cover (as in most other species of lizards). Risks of predation are likely to be increased during long periods of exposure while foraging in the open. A reluctance to move, even when actually threatened by a potential predator, could well be advantageous; movement might attract attention of predators and negate the advantage of concealing coloration and contour. Such decreased movement doubtless contributes to the observed high variance in body temperature of Phrynosoma platyrhinos, which is significantly greater than that of all other species of sympatric lizards. Phrynosoma platyrhinos are also active over a longer time interval than any sympatric lizard species. Wide fluctuations in horned lizard body temperatures under natural conditions presumably reflect both the long activity period and perhaps their reduced movements into or out of the sun and shade (most of these lizards are in the open sun when first sighted). More time is thus made available for activities such as feeding. A foraging anteater must spend considerable time feeding. Food specialization on ants is economically feasible only because these insects usually occur in a clumped spatial distribution and hence constitute a concentrated food supply. To make use of this patchy and spatially concentrated, but at the same time not overly nutritious, food supply, P. platyrhinos has evolved a unique constellation of adaptations that include a large stomach, spiny body form, an expanded period of activity, and "relaxed" thermoregulation (eurythermy). The high reproductive investment of adult horned lizards is probably also a simple and direct consequence of their robust body form (Pianka and Parker, 1975; Vitt and Congdon, 1978). Lizards that must be able to move rapidly to escape predators, such as racerunners (Cnemidophorus), would hardly be expected to weight themselves down with eggs to the same extent as animals like horned lizards that rely almost entirely upon spines and camouflage to avoid their enemies.  Phylogenetic Constraints and Evolutionary Pathways Ecologists have only recently begun to adopt a phylogenetic perspective. Related species do not constitute independent observations due to their shared evolutionary histories (Felsenstein 1985). Phylogenetic relationships among members of a monophyletic group must be known in order to interpret evolution, comparative anatomy and ecology (Felsenstein 1988; Huey and Bennett 1986).With a known phylogeny, the evolution of niche transitions can be plotted. Huey and Bennett (1987) and Garland et al (1991) have developed approaches to examine the influence of phylogeny on coadaptation of thermal physiology in some Australian skinks, including several species of Ctenotus. Their work provides a foundation for a study of evolution within the genus Ctenotus and suggests a hypothesis: the Ctenotus adaptive radiation was partially a consequence of the evolution of higher body temperatures (most, but not all, Ctenotus, display appreciably higher body temperatures than related skinks). Given a phylogenetic perspective, anatomical and ecological convergences can be identified. With a known phylogeny, the evolution of niche transitions can be plotted. Comprehensive study of comparative anatomy and ecology of a species-rich genus with a known phylogeny could actually enable prediction of the probable ecologies of unstudied members of that genus, which could then be tested by examination in the field. Such efforts to ascertain evolutionary pathways, as well as the extent to which phylogeny has constrained morphology and ecology, should prove to be most instructive. I am undertaking such an analysis of the speciose Australian skink genus Ctenotus. Towards a Periodic Table of Lizard Niches: Reducing Dimensionality To order and classify natural phenomena, chemists invented the well-known periodic table of the elements, which allowed them to predict unknown elements as well as certain of their chemical properties and led to our understanding of electron shells. Something analogous, but much more complex than the periodic table of the elements, a "periodic table of niches" might be possible in ecology. Of course, nothing about ecological niches is as simple or discrete as the number of electrons in the outer shell of a chemical element, but most aspects of ecological niches are multidimensional and more continuous. Powerful multivariate statistical techniques, such as principal components analysis, have been designed to represent the same data but in a new set of orthogonal axes with reduced dimensionality. For original variates that are variously correlated, the first principal component (PC1) is the line through n-dimensional hyperspace that reduces variance by the greatest amount, leaving the smallest residual (unexplained) variance. The second PC is constrained to be at right angles to the first, and reduces the residual variance as much as possible. Likewise for the third PC which is constrained to be a right angles to both the first and second PCs, and so on. Principal components represent combinations of the original dimensions, weighted according to their contribution. With highly correlated data sets, dimensionality can often be greatly reduced. For example, 96% of the variance in a 10-dimensional morphometric hypervolume was "explained" by just the first three principal components (Pianka 1986), leaving a residual "unexplained" variance of just 4%. Over 20 years ago, I constructed a very primitive example of a periodic table of niches (Pianka 1974). Dietary niches repeat themselves in organisms of different sizes that are relatively more or less r- and K-selected. An aphid is more like a lemming and a mantid more like a weasel in their trophic niche, whereas in terms of body size and position on the r-K selection continuum, the aphid and mantid are relatively alike, as are the lemming and the weasel. Other niche dimensions, such as diurnal and nocturnal time of activity, could also be used to construct similar but different periodic tables. My suggestion that a periodic table of lizard niches might actually be possible (Pianka 1986) was dismissed as "somewhat fanciful" by Gregory (1989). Today I have outlined most of the dimensions required to construct a periodic table of lizard niches. Even though I have not yet been able to achieve this goal, I still hold high hopes that lizard niches can eventually be classified in a space of moderately low dimensionality using axes such as the thermoregulator-thermoconformer continuum (discussed above) and Dunham and Miles' (1985) discriminant function axis linking mode of foraging to body size and reproductive tactics. Unfortunately, only 13 of the 82 species used to generate the first axis are included among the 91 species used to generate the second axis. Until more data on lizard niches are gathered, such an analysis would be premature. Literature Cited Arnold, E. N. 1988. Caudal Autotomy as a defense. Chapter 3 (pp. 235-273) in Gans, C. and R. B. Huey (eds.) Biology of the Reptilia, Volume 16, Ecology B. Defense and Life History. Alan R. Liss, Inc., New York. Bartholomew, G. A. 1972. Body temperature and energy metabolism. Chapter 8 (pp. 298-368) M. S. Gordon (ed.), Animal physiology: Principles and adaptations. Macmillan, New York. Bennett, A. F. and K. A. Nagy. 1977. Energy expenditure in free-ranging lizards. Ecology 58: 697-700. Cowles, R. B. and C. M. Bogert. 1944. A preliminary study of the thermal requirements of desert reptiles. Bull. Amer. Museum Natural History 83: 261-296. Dunham, A. E. and D. B. Miles. 1985. Patterns of covariation in life history traits of squamate reptiles: the effects of size and phylogeny reconsidered. American Naturalist 126: 231-257. Felsenstein, J. 1985. Phylogenies and the comparative method. Amer. Natur. 125: 1-15. Felsenstein, J. 1988. Phylogenies and quantitative characters. Annual Review of Ecology and Systematics 19: 445-471. Finlayson, H. H. 1943. The Red Centre. Angus and Robertson, Sydney. Garland, T., Huey, R. B., and A. F. Bennett. 1991. Phylogeny and coadaptation of termal physiology in lizards: a reanalysis. Evolution 45: 1969-1975. Gregory, P. T. 1989. Review of "Ecology and Natural History of Desert Lizards." Canadian Field-Naturalist 102: 743-744. Hamilton, W. J., III. 1973. Life's color code. McGraw-Hill, New York. Huey, R. B. and A. F. Bennett. 1986. A comparative approach to field and laboratory studies in evolutionary biology. Chapter 6 (pp. 82-98) in M. E. Feder and G. V. Lauder (eds.), Predator-Prey Relationships. Univ. Chicago Press, Chicago. Huey, R. B. and A. F. Bennett. 1987. Phylogenetic studies of coadaptation: preferred temperatures versus optimal performance temperatures of lizards. Evolution 41: 1098-1115. Huey, R. B. and E. R. Pianka. 1977a. Seasonal variation in thermoregulatory behavior and body temperature of diurnal Kalahari lizards. Ecology 58: 1066-1075. (With an Appendix by J. A. Hoffman.) Huey, R. B. and E. R. Pianka. 1977b. Natural selection for juvenile lizards mimicking noxious beetles. Science 195: 201-203. Huey, R. B. and E. R. Pianka. 1981. Ecological consequences of foraging mode. Ecology 62: 991-999. Huey, R. B. and M. Slatkin. 1976. Costs and benefits of lizard thermoregulation. Quarterly Review of Biology 51: 363-384. Huey, R. B., E. R. Pianka, M. E. Egan, and L. W. Coons. 1974. Ecological shifts in sympatry: Kalahari fossorial lizards (Typhlosaurus). Ecology 55: 304-316. Luke, C. 1986. Convergent evolution of lizard toe fringes. Biol. J.Linn. Soc. 27: 1-16. MacArthur, R. H. and E. R. Pianka. 1966. On optimal use of a patchy environment. American Naturalist 100: 603-609. Magnusson, W. E., L. J. de Paiva, R. M. da Rocha, C. R. Franke, L. A. Kasper and A. P. Lima. 1985. The correlates of foraging mode in a community of Brazilian lizards. Herpetologica 41: 324-332. McLaughlin, R. L. 1989. Search modes of birds and lizards: evidence for alternative movement patterns. The American Naturalist 133: 654-670. Nagy, K. A., R. B. Huey, and A. F. Bennett. 1984. Field energetics and foraging mode of Kalahari lacertid lizards. Ecology 65: 588-596. Perry, G., I. Lampl, A. Lerner, D. Rothenstein, E. Shani, N. Sivan and Y. L. Werner. 1990. Foraging mode in lacertid lizards: variation and correlates. Amphibia-Reptilia 11: 373-384. Pianka, E. R. 1966. Convexity, desert lizards, and spatial heterogeneity. Ecology 47: 1055-1059. Pianka, E.R. 1969. Notes on the biology of Varanus caudolineatus and Varanus gilleni. Western Australian Naturalist 11: 76-82. Pianka, E.R. 1970. Comparative autecology of the lizard Cnemidophorus tigris in different parts of its geographic range. Ecology 51: 703-720. Pianka, E. R. 1973. The structure of lizard communities. Annual Review of Ecology and Systematics 4: 53-74. Pianka, E. R. 1974. Evolutionary Ecology. First Edition. Harper and Row, New York. Pianka, E. R. 1981. Competition and niche theory. Chapter 8 (pp. 167-196) in: "Theoretical Ecology, Second Edition," R. M. May, ed. Blackwell. Pianka, E. R. 1985. Some intercontinental comparisons of desert lizards. National Geographic Research 1: 490-504. Pianka, E. R. 1986. Ecology and Natural History of Desert Lizards. Analyses of the Ecological Niche and Community Structure. Princeton University Press, Princeton, New Jersey. 208 pp. Pianka, E. R. 1989. Desert lizard diversity: additional comments and some data. American Naturalist 134: 344-364. Pianka, E.R., R.B. Huey and L.R. Lawlor. 1979. Niche segregation in desert lizards. Chapter 4 (pp. 67-115) in "Analysis of Ecological Systems," D.J. Horn, R. Mitchell, and G.R. Stairs, eds. Ohio State University Press, Columbus. Pianka, E.R. and W.S. Parker. 1975. Ecology of horned lizards: A review with special reference to Phrynosoma platyrhinos. Copeia 1975: 141-162. Pianka, E.R. and H.D. Pianka. 1976. Comparative ecology of twelve species of nocturnal lizards (Gekkonidae) in the Western Australian desert. Copeia 1976: 125-142. Pietruszka, R. D. 1986. Search tactics of desert lizards: how polarized are they? Animal Behavior 34: 1742-1758. Rand, A. S. 1967. Predator-prey interactions and the evolution of aspect diversity. Atas do Simposio sobre a Biota Amazonica 5: 73-83. Robinson, M. D. and A. B. Cunningham. 1978. Comparative diet of two Namib desert sand lizards (Lacertidae). Madoqua 11: 41-53. Rosen, R. 1967. Optimality principles in biology. Plenum, New York. Schall, J. J. and E. R. Pianka. 1978. Geographical trends in numbers of species. Science 201: 679-686. Schall, J. J. and E. R. Pianka. 1980. Evolution of escape behavior diversity. American Naturalist 115: 551-566. Schoener, T. W. 1979. Inferring the properties of predation and other injury-producing agents from injury frequencies. Ecology 60: 1110-1115. Shine, R. and J. J. Bull. 1979. The evolution of live-bearing in lizards and snakes. American Naturalist 113: 905-923. Simpson, E. H. 1949. Measurement of diversity. Nature 163: 688. Vitt, L. J. and J. D. Congdon. 1978. Body shape, reproductive effort, and relative clutch mass in lizards: resolution of a paradox. American Naturalist 112: 595-608. Vitt, L. J., J. D. Congdon, and N. Dickson. 1977. Adaptive strategies and energetics of tail autotomy in lizards. Ecology 58: 326-337. Williams, E. E. 1983. Ecomorphs, faunas, island size, and diverse end points in island radiations of Anolis. Chapter 15 (pp. 326-370) in R. B. Huey, E. R. Pianka, and T. W. Schoener (eds.) Lizard Ecology: Studies of a Model Organism. Harvard University Press. Zweifel, R. G. and C. H. Lowe. 1966. The ecology of a population of Xantusia vigilis, the desert night lizard. American Museum Novitates 2247: 1-57. Books by Eric R. Pianka 
Return to Pianka lab page |