<i>Moloch</i>
Australia's Thorny Devil
© by Eric R. Pianka

Australia is home to many magnificent animals, but one of the most bizarre of all is a spiny lizard known as the thorny devil or mountain devil. A thorny devil was first exhibited in London by John Gould in 1840. This spectacular specimen was illustrated by John Gray in 1841 who gave the thorny devil its official scientific name, the Latin binomial Moloch horridus. Gray named this species of lizard after a fearful Canaanite god called Moloch. Dr. Gray had in mind Milton's Moloch, a horrid king smeared with the blood of human sacrifice. The Latin specific name horridus means rough or bristly, or secondarily dreadful. Close ups of thorny devils are often shown in movies that are filmed in inland Australia, and most people have a mental image of these lizards as gigantic dreadful monsters -- in fact, thorny devils are only moderate-sized lizards about 4-6" long.
Saville-Kent (1897) was among the first people to keep thorny devils in captivity in Australia,
observing their behavior in detail. He discovered that they fed almost exclusively on ants.
Saville-Kent noted the striking morphological similarity between the thorny devil and North
American horned lizards, genus Phrynosoma. Thorny devils are agamid lizards, very distantly related to horned lizards, which are phrynosomatids. 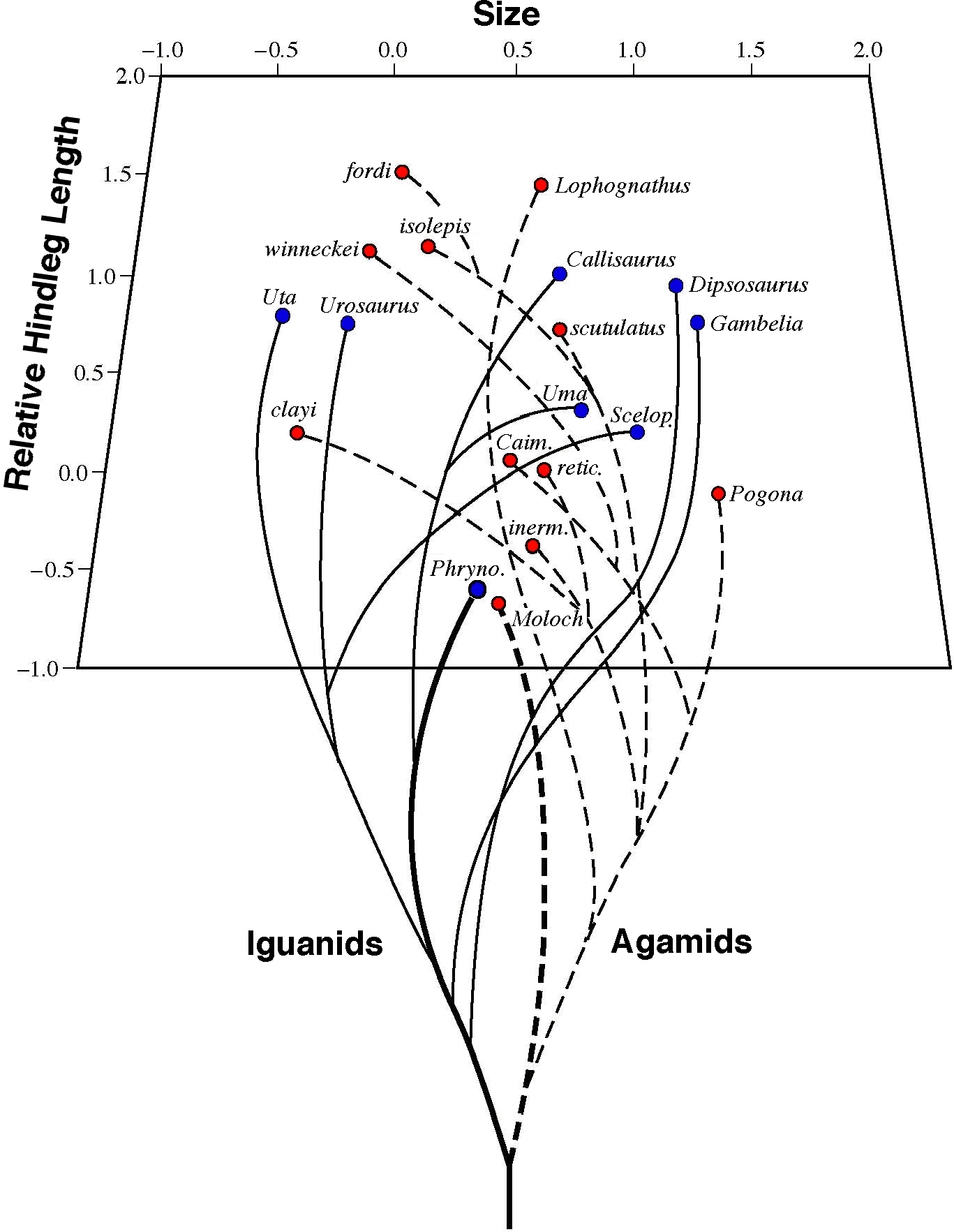 On the basis of this similarity in body form, Saville-Kent predicted that horned lizards would be found to be an ant eaters -- his prediction proved to be entirely correct. A multivariate morphometric analysis demonstrates that thorny devils and horned lizards are actually anatomically closer to one another than either species is to another member of its own lizard fauna, which are much more closely related phylogenetically (Pianka 1986, 1994). On the basis of this similarity in body form, Saville-Kent predicted that horned lizards would be found to be an ant eaters -- his prediction proved to be entirely correct. A multivariate morphometric analysis demonstrates that thorny devils and horned lizards are actually anatomically closer to one another than either species is to another member of its own lizard fauna, which are much more closely related phylogenetically (Pianka 1986, 1994).
Such organisms that fill similar ecological niches in different, independently-evolved, biotas are known as "ecological equivalents". Convergent evolution of this sort is most interesting because it implies that similar selective pressures have resulted in the same solutions to adapt to particular environmental situations. The very existence of such convergent species pairs indicates that evolutionary pathways can be predictable and repeatable. I studied North American desert horned lizards in detail during 1962-65. I went to Australia in 1966 with the hope of documenting such evolutionary convergence in detail and also perhaps contributing new ideas about the vital process of natural selection. After travelling over 12,000 kilometers over several months in arid Australia without finding a single thorny devil, I began to despair that I would never be able to find one. One day I got lucky and spotted a thorny devil crossing the road. Like a horned lizard, this thorny devil didn't move and was easy to catch. I took it to some soft sand and backed off a ways and waited, allowing it to walk and leave a trail. I studied its track with care and eventually learned how to find these elusive lizards by following their delicate footprints. This is far from easy and even now I often cannot locate individuals of this species. Tracks that lead into a large bush or a messy area of litter and bushes cannot be followed, and one must try to find the animal by sight.

Once, at a very remote site deep in the Great Victoria Desert where thorny devils had never before been recorded, I had spent most of the day trying to find an animal whose track went around and around in a large figure eight. We were running out of food and water and had to return to civilization the next day to resupply. It was late in the day and the sun would soon set. My ex-wife Helen and I were standing a couple meters apart at the center of the figure eight, shaking our heads, wondering where the thorny devil was, when suddenly both of us saw the lizard at the same instant, in its dark green color phase hunkered down right between us. Needless to say, thorny devils are exceedingly well camouflaged and very difficult to see, even when you have the correct search image in mind!
Distribution and Habitat
Thorny devils are found through most of arid inland Australia, particularly on sandy soils, but they seldom occur on stony soils. Thorny devils are found in two quite different habitats: spinifex-sandplain and sandridge deserts of the interior and the mallee belt of southern South Australia and southwestern Western Australia. The geographic distribution of thorny devils corresponds more closely to the distribution of sandy and sandy loam soils than to any climatological field.
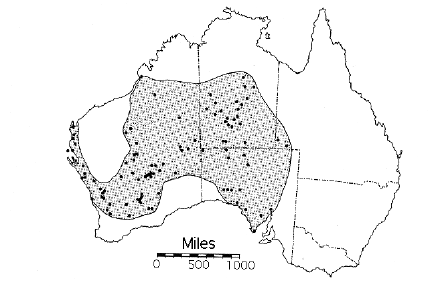
Figure 1. Geographic distribution of thorny devils.
|
Food and Feeding Biology
Thorny devils are obligate ant specialists, eating virtually nothing else. They will consume several species of ants, but are especially partial to very small Iridomyrmex ants, especially Iridomyrmex flavipes. Feeding rates have been estimated at from 24 to 45 ants per minute. Occasional objects such as small stones, sticks, tiny flowers and small insect eggs are also ingested -- these are probably objects being carried by ants and are eaten only accidentally. Large numbers of ants are eaten per meal by an individual thorny devil (estimates range from 675 to 1000-1500 to 2500)
Fecal pellets of thorny devils are very distinctive: black, glossy, perfect prolate spheroids. These are often found in neat piles either in the open or amongst sparse vegetation. Individuals have specific defecation sites, separate from their basking and feeding sites. Tracks and accumulations of fecal matter indicate that thorny devils often return to such spots several days in succession.
Water Uptake
Thorny devils have a hygroscopic system of grooves in their skin that lead to the corners of their mouth. Bentley and Blumer (1962) showed that thorny devils take up water by means of capillary action via these grooves. Thorny devils use a gulping oral mechanism to move water along the grooves and into their mouths. Thorny devils can actually drink water from dew that falls on their backs and they can gain as much as a gram of water in a rainstorm.
False Head and Predation
Thorny devils posses a curious knob-like spiny appendage on the backs of their necks, which has sometimes been likened to a false head. When threatened, the lizards tuck their real heads down between their forelegs leaving this false head in the position of their real head. This would certainly make them difficult for most predators to swallow, even a snake. Once I caught a thorny devil that had a damaged knob which looked as if a mammalian predator had chewed on it. When disturbed, thorny devils will also inflate themselves with air, puffing up like little puffer fish. This is presumably another anti-predator defense.

Thorny devils walk slowly with a jerky motion, and they often freeze in place while walking, frequently with a foot off the ground. This probably helps to conceal a lizard caught out in the open. They can also change color rapidly -- when warm and active, they are usually a pale yellow and red, but when alarmed or when they are cold, they are dark olive drab. Despite their camouflage and thorny spines, thorny devils are not immune to predation. Australian aborigines and bustards, both visual predators, have been observed to prey upon thorny devils. Once, while tracking a medium-sized thorny devil, I encountered a sand goanna, Varanus gouldii, which I collected and preserved for later analysis in the lab. I gave up on finding that thorny devil -- its tracks just vanished into thin air. Six months later back in the USA, while dissecting the monitor lizard, I found that thorny devil in its stomach! I remain amazed that the thorny devil's spines didn't puncture the monitor's thin stomach wall. I also found another small thorny devil in the stomach of a racehorse monitor lizard, Varanus tristis. Raptors are also probable predators of thorny devils.
Parasites
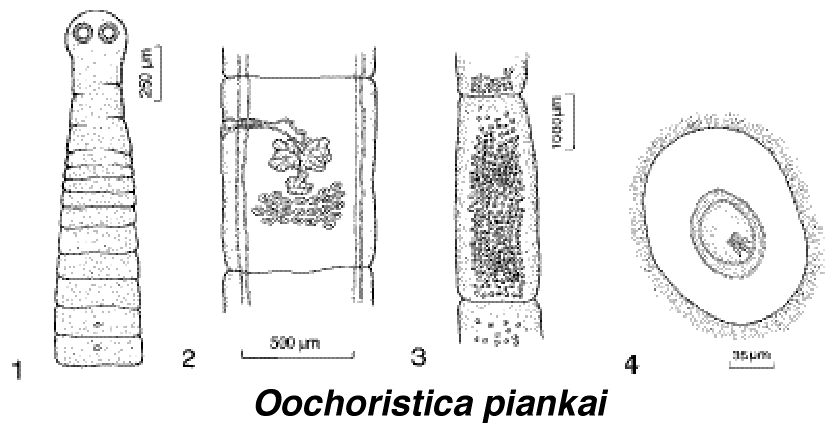
Thorny devils are usually heavily parasitized, with almost all individuals having many nematode worms, Parapharyngodon kartana and Abbreviata sp., in their intestinal tracts. These parasites probably exploit ants and termites as intermediate hosts and various species of lizards as their final hosts. In addition, a new species of tapeworm the cestode Oochoristica piankai, was recently described from the small intestines of thorny devils (Bursey et al. 1996).
Thermoregulation
Thorny devils are heliotherms, relying on solar energy to raise their body temperatures above ambient temperatures. Body temperature varies directly with air temperature. Average body temperature of 83 active thorny devils is 33.3°C (Standard deviation = 2.9).
Size, Growth and Age
Adult female thorny devils are larger and stouter than adult males. Adult females range from 80 to 110 mm in snout-vent length and weigh from 33 to 88.7 gms, whereas adult males are all under 96 mm in snout-vent length and never weigh more than 49 gms. There is no noticeable size difference between hatchling males and females. Sporn (1955, 1958, 1965) kept captive individuals for 5-8 years and showed that females and males grow at about the same rate during their first year, but after that, females grow faster than males. Thorny devils continue to grow until at least their fifth year. Sporn kept some captives for up to 8 years and considered it highly likely that thorny devils live up to 20 years in nature. Someone should undertake a detailed long-term mark and recapture study of these interesting lizards.
Seasonal Activity and Reproduction
Thorny devils display a bimodal seasonal pattern of activity. These lizards move little during the coldest winter months (June and July) or during the hottest summer months (January and February). Thorny devils are active during a three-month autumnal period (March, April, and May) and during a five month activity period that spans late winter, spring and early summer (August through December), during which mating and egg deposition take place. During hot summer days, thorny devils retreat into shallow underground burrows dug by themselves.
Thorny devils are sedentary during summer and autumn, with an individual's movements generally being restricted to an area about 20-30 feet on a side. Within this area, there are one or more ant trails, a defecation site, and several small bushes with scattered dead leaves and/or loose Triodia spinifex tussocks beneath them. Thorny devils spend the night and the heat of mid-day within the protective cover of such shrub complexes (sometimes actually within a loose spinifex tussock), and take refuge in them upon the approach of a potential predator. Tracks and observations on several focal individuals suggest the following general pattern of daily activity: The lizard first walks a short distance out into the sun from the shrub(s) under which it has spent the night. After basking and bellying down in the sand, the lizard attains an active body temperature and begins to move. Often an individual then walks 5-10 m to its defecation site, defecates, and returns by nearly the same route to the shrub complex, stopping to feed at an ant trail somewhere along the way. Mating has been observed in the autumn, which suggests that thorny devils may have a mechanism of sperm storage.
In contrast to the relatively sedentary summer-autumn existence, thorny devils move over much greater distances during August and September when most mating occurs. One encounters many fresh cross-country tracks in early spring, relatively straight tracks extending for linear distances of several hundred feet or more. Such increased movements doubtlessly increase the probability of contact between individuals. On 25 August 1967, we observed one small aggregation of two males and two females in an area immediately adjacent to and beneath a large shrub. Tracks clearly indicated that all four individuals had travelled varying distances (150-250 ft.) toward and converged upon this shrub from different directions, all in less than 24 hr (strong winds had erased all tracks the day before).
Evidence of a similar gathering was observed at another study site, also in August, although we could only find two of these individuals, both of which were males. Tracks indicated the presence of two other much larger individuals (probably females) and showed that considerable mingling had occurred. Despite concerted efforts, we found no evidence of such aggregations at any other time. The above two cases are striking because of their rarity and simultaneous occurrence and were almost certainly mating contacts.
In mid September, we introduced a male thorny devil into an enclosure with a single female. Neither lizard appeared to be aware of the other for long periods of from 15 to 60 minutes. Then, the male would suddenly move in the direction of the female and rapidly bob its head a number of times. If the female remained stationary, the male would attempt to mount her from behind, with one hind leg still on the ground. The female was unreceptive and threw the male off her back several times by a quick sideways roll and then moved quickly away from the male.
Eggs are laid from mid September through late December. Usually only a single clutch is laid per year. Clutch size in thorny devils varies from three to ten with a mode of eight eggs per clutch. Seven clutches had incubation times of from 90 to 132 days (mean 118 days).
During the austral spring of 1995, with the assistance of several colleagues (Graham Thompson, Gretchen Pianka, and Muriel DenBoer),
I undertook radiotelemetric studies of movements of thorny devils. Small transmitters were attached externally. We "wired up" 9 thorny devils (3 males and 6 females) and followed their movements daily from mid-September through early November 1995. Two males disappeared suddenly and were probably eaten by predators, most likely by Australian bustards, which were abundant in the area. One female and one male undertook long distance cross country treks, travelling total distances of a kilometer over a period of several days. The other thorny devils were fairly sedentary, moving only short distances.

Three of the female thorny devils excavated nest chambers and laid clutches of eggs (19 Sept., 3 Oct., 12-13 Nov.). All three nesting burrows were located on the southern side of the same sandridge (two of the females crossed over the ridge from the north side prior to digging). Females spent several days excavating their nesting burrows, using their hindfeet, which kick the sand out of the tunnel. Their nesting burrows went down about 9" and then took an abrupt right angle turn. After laying eggs, the females carefully backfilled these tunnels, smoothing over the sand and leaving no trace of a burrow.
One female backfilled her burrow during the night! These three females invested heavily in their clutches, with relative clutch masses (total weight of eggs divided by female total weight before oviposition) of 34.2%, 41.7%, and 40.9%, respectively.
We erected wire enclosures around the first two nests to exclude predators and to hold in the baby lizards after hatching so that they could be weighed, measured, and photographed. The third nest fell victim to predation, being dug up, most probably by a sand goanna, Varanus gouldi. Nests were checked daily until early February. Hatchlings emerged from the first clutch on 20-21 Jan. 1996 and from the second clutch on 7 Feb. 1996 (Figure). All hatchlings emerged from a single foot-long diagonal tunnel to the surface. Hatchlings weighed an average of 1.8 gms and measured from 63 to 65 mm in total length (snout to tip of tail).
Shortly after hatching, I carefully excavated both nesting burrows -- both had air-filled chambers about 3" x 5" x 6" about 9" below the hard-packed sand's surface (after hatching, air temperature in both these chambers was about 31°C). No eggshells were present, suggesting that hatchlings had eaten their egg cases. This could add to their body mass and provide hatchlings with calcium and other materials to support early growth. I know of no other squamate reptile that deposits its eggs in an air-filled chamber or consumes its own eggshells. Egg shell consumption could be facilitated by the air chamber -- other squamates simply cover their eggs with damp sand which would make it nearly impossible for a hatchling to find and eat its own egg shell.
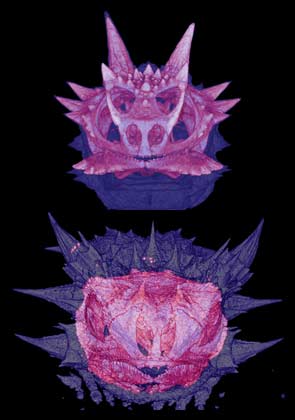 Thorny devils possess more spines than horned lizards, and they are at least as sharp.
Most of their spines, including the false head, are entirely boneless. Moloch is nevertheless
stunningly spiny via dermal armor alone, demonstrating that convergent evolution need not exploit
the same mechanisms to achieve a given anatomical endpoint. Horned lizard skulls possess boney
spines inside each of the many spines on their heads (top, left),
but the spines of thorny devils are constructed from keratin rather than bone (bottom, left).
Each has evolved spines independently using different anatomical pathways, an example of
convergent evolution in these ecological equivalents. To see cat scans of Moloch compared with those of Phrynosoma,
go to Tim Rowe's Digimorph website at http://www.digimorph.org/specimens/Moloch_horridus/whole.
Thorny devils possess more spines than horned lizards, and they are at least as sharp.
Most of their spines, including the false head, are entirely boneless. Moloch is nevertheless
stunningly spiny via dermal armor alone, demonstrating that convergent evolution need not exploit
the same mechanisms to achieve a given anatomical endpoint. Horned lizard skulls possess boney
spines inside each of the many spines on their heads (top, left),
but the spines of thorny devils are constructed from keratin rather than bone (bottom, left).
Each has evolved spines independently using different anatomical pathways, an example of
convergent evolution in these ecological equivalents. To see cat scans of Moloch compared with those of Phrynosoma,
go to Tim Rowe's Digimorph website at http://www.digimorph.org/specimens/Moloch_horridus/whole.
References
Bentley, P. J. and F. C. Blumer. 1962. Uptake of water by the lizard, Moloch horridus. Nature 194: 699-700.
Download pdf.
Bransdon, J. 1952. I adopted three dragons. Walkabout 18 (8): 19-20.
Bursey, C. R., S. R. Goldberg, and D . N. Woolery. 1996. Oochoristica piankai sp. n. (Cestoda: Linstowiidae) and other Helminths of Moloch horridus (Sauria: Agamidae) from Australia. J. Helminthol. Soc. Was. 63: 215-221.
Clemente, C. J., G. G. Thompson, P. C. Withers and D. Lloyd. 2004. Kinematics, maximal metabolic rate, sprint and endurance for a slow-moving lizard, the thorny devil (Moloch horridus). Australian Journal of Zoology 52: 487-503.
Greer, A. E. 1989. The biology and Evolution of Australian Lizards. Surrey Beatty and Sons Pty Ltd.
Jones, H. I. 1995. Gastric nematode communities in lizards from the Great Victoria Desert, and an hypothesis for their evolution. Australian Journal of Zoology 43: 141-164.
Pianka, E. R. 1986. Ecology and Natural History of Desert Lizards. Analyses of the Ecological Niche and Community Structure. Princeton University Press, Princeton, New Jersey.
Pianka, E. R. 1994. Biodiversity of Australian desert lizards. In C.-I. Peng and C. H. Chou (eds.) Biodiversity and Terrestrial Ecosystems. Institute of Botany, Academica Sinica, Monograph Series No. 14, pp. 259-281.
Pianka, E. R. 1997.
Australia's thorny devil. Reptiles 5 (11): 14-23. Download pdf
Pianka, E. R. and W. L. Hodges . 1998.
Horned lizards. Reptiles 6 (6): 48-63.
Pianka, E. R. and W. S. Parker. 1975. Ecology of horned lizards: A review with special
reference to Phrynosoma platyrhinos. Copeia 1975: 141-162.
Download pdf
Pianka, E. R. and H. D. Pianka. 1970. The ecology of
Moloch horridus (Lacertilia: Agamidae) in Western
Australia. Copeia 1970: 90-103. Download pdf
Pianka, G. A., E. R. Pianka, and G. G. Thompson. 1996. Egg laying by thorny devils (Moloch
horridus) under natural conditions in the Great Victoria desert. J.
Roy. Soc. Western Australia 79: 195-198. Download pdf
Pianka, G. A., E. R. Pianka, and G. G. Thompson. 1998. Natural history
of thorny devils Moloch horridus (Lacertilia: Agamidae) in the
Great Victoria desert. J. Royal Society of Western Australia 81: 183-
190. Download pdf
Pianka, E. R. 2003. "Moloch horridus" (On-line), Digital Morphology.
Pianka, E. R. 2003. "Moloch horridus" (On-line), Digital Morphology.
Saville-Kent, W. 1897. The Naturalist in Australia. London.
Sporn, C. C. 1955. The breeding of the mountain devil in captivity. Western Australian Naturalist 5: 1-5.
Sporn, C. C. 1958. Further observations on the mountain devil in captivity. Western Australian Naturalist 6: 136-137.
Sporn, C. C. 1965. Additional observations on the life history of the mountain devil, Moloch horridus, in captivity. Western Australian Naturalist 9: 157-159.
P. C. Withers and C. R. Dickman.1995. The role of diet in determining water, energy and salt uptake in the thorny devil Moloch horridus (Lacertilia: Agamidae). J. Roy. Soc. Western Australia 78: 3-11.
Withers P.C. and S. D. Bradshaw. 1995. Water and energy balance of the thorny devil Moloch horridus: is the devil a sloth? Amphibia-Reptilia 16:4754.
|
| | |






