|
Parasitism and Predator Escape Tactics |
|
Parasites can have rather profound effects on the ecology of their hosts. Effects of the malarial parasite Plasmodium mexicanus on members of a population of Sceloporus occidentalis lizards were examined in California by Schall (1982). About one-third to 40 percent of the male lizards were infected, whereas only 15 to 30 percent of the female lizards had malaria (percentage infected increases with age). Blood hemoglobin levels were lower in parasitized lizards than in unparasitized ones. When lizards were at rest, oxygen consumption rates did not differ between the two groups, but during maximal activity, infected animals had significantly lower metabolic rates. Moreover, both the capacity for aerobic metabolism and running stamina (as measured in an oval track) were reduced in infected lizards as compared to controls. Parasitized lizards also tended to have smaller fat reserves and smaller clutch sizes than noninfected ones. Effects of infection by malarial parasites might be subtle and hosts might appear healthy, but these parasites seem to reduce host fitness substantially.  Warningly colored red eft salamander. In another study, Schall (1992) demonstrated that another species of malarial parasite allowed coexistence of two species of Caribbean Anolis lizards (in the absence of the parasite only one species of lizard occurs, but if this species of lizard is parasitized, the other lizard species can coexist with it). 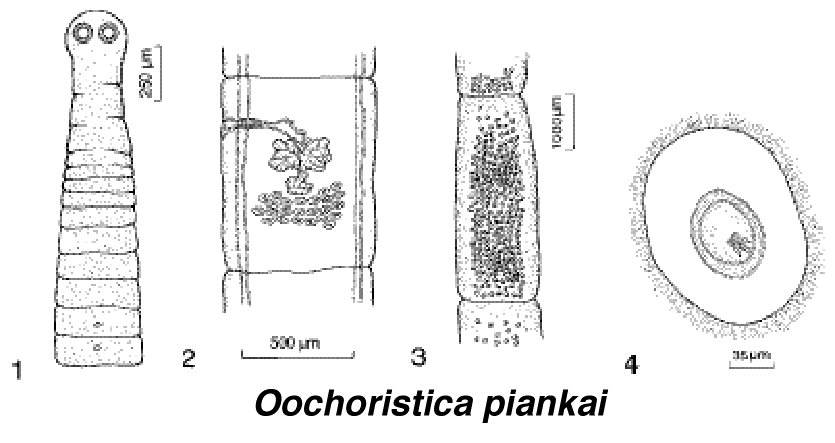 Recently described cestode parasite of Moloch, named Oochoristica piankai. From left to right: (1) anterior portion, showing scolex and proglottids, (2) mature proglottid, (3) gravid proglottid, (4) uterine capsule with onchosphere. The generic name Oochoristica translates as "hairy egg." Oochoristica is a cosmopolitan genus of cestodes, with 74 described species from Africa, Australia, North America, and Eurasia. All are parasites of various reptiles including both lizards and snakes. Lizard Tails Lizard tails have diversified greatly and serve a wide variety of functions for their possessors. Many climbing species, such as the Australian sandridge agamid Gemmatophora longirostris, have evolved extraordinarily long tails which serve as effective counterbalances. Long tails enable lizards to raise their forelegs up off the ground and to run on their hind legs alone (bipedality is a faster means of locomotion than tetrapodality). Prehensile tails are used as a fifth leg in climbing by some arboreal lizard species like some geckos (e.g., Diplodactylus elderi) and by the true chameleons (Chameleo dilepis) of the Kalahari.  <--- Right: Spinifex gecko Strophurus elderi
<--- Right: Spinifex gecko Strophurus elderi
Below: Kalahari Chameleo dilepis 
 Another Australian desert lizard with a similar yet different tail tactic is the climbing skink
Egernia depressa.
This Australian arboreal skink (Egernia depressa) uses its spiny tail to block off crevices in rocks and trees. These lizards wedge themselves into tight crevices in mulga tree hollows (and rocks), blocking off the entrance with their strong and very spiny tails. Spinily-armored tails are used by numerous other species of lizards in a similar fashion, including the Mexican iguanid Enyaliosaurus clarki and the Saharan agamid Uromastix acanthinurus.
Another Australian desert lizard with a similar yet different tail tactic is the climbing skink
Egernia depressa.
This Australian arboreal skink (Egernia depressa) uses its spiny tail to block off crevices in rocks and trees. These lizards wedge themselves into tight crevices in mulga tree hollows (and rocks), blocking off the entrance with their strong and very spiny tails. Spinily-armored tails are used by numerous other species of lizards in a similar fashion, including the Mexican iguanid Enyaliosaurus clarki and the Saharan agamid Uromastix acanthinurus.
 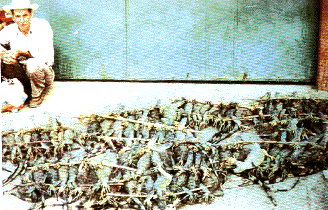 A pile of iguanas for sale in a Central American marketplace. Lizards are hogtied with their jaws sewn shut. They are kept alive so that people without refrigeration can eat them later at will. One most unfortunate species' scientific name is Ctenosaura delicatissima. Certain small predators, such as the pygmy varanids Varanus gilleni and V. caudolineatus, may actually "harvest" the exceedingly fragile tails of geckos that are too large to subdue intact (Pianka 1969). Tails of many, but by no means all, lizards break off easily. Indeed, some species can actually lose their tails voluntarily with minimal external force in a process known as autotomy (Arnold 1988). Freshly dismembered tails or pieces thereof typically thrash around wildly, presumably attracting a predator's attention while the recent owner quietly slips away unnoticed (Vitt et al. 1977). Some skinks, including many Ctenotus, return to the site where their tail was lost and swallow the remains of their own tail! Few, if any, other vertebrates display auto-amputation and self cannibalism. Many such lizards possess special adaptations for tail loss, including weak fracture planes within each tail vertebra, muscular attachments that facilitate autotomy and tail movement after dismemberment, as well as mechanisms for rapidly closing off blood vessels and healing. Losing its tail has surprisingly little effect on a lizard, as individuals often resume basking and foraging as if nothing had happened within minutes. In such lizard species, of course, tails are quickly regenerated from the stub. Although regrown tails are occasionally almost indistinguishable from the original externally, their internal support structure is cartilaginous rather than bony. Not all lizard tails are easily broken, however. Whereas most phrynosomatids have fragile tails, their close relatives the agamids generally do not. Tails of varanids and true chameleons do not break easily either. Lizards with such tough tails usually cannot regenerate a very complete tail if their original should happen to be lost. The evolutionary bases for these differences, sometimes between fairly closely-related groups of lizards, are evasive. Arnold (1988) argues that easy tail loss is a primitive trait among lizards which has been lost but regained repeatedly among various lizard lineages.  The potential of tail break frequency as an index to the intensity of predation on lizard populations was first pointed out by J.B.S. Haldane. It has since been used to attempt to estimate the amount of predation, although there are serious problems and limitations with the procedure (Schoener 1979). Efficient predators that leave no surviving prey obviously will not produce broken tails, but nevertheless may exert substantial predation pressures: broken and regenerated tails may therefore reflect lizard escape ability or predator inefficiency better than intensity of predation. Predator densities increase from north to south in western North America (Pianka 1986; Schall and Pianka 1980). Correlated with this latitudinal increase in predation, frequencies of broken and regenerated tails are higher at southern sites than at northern localities among four of the five widely-distributed lizard species. In the well-studied species Cnemidophorus tigris, frequency of broken tails decreases with latitude (Pianka 1970); moreover, diversity of predator escape behaviors utilized among members of these various Cnemidophorus populations also increases with the frequency of broken and regenerated tails (Schall and Pianka 1980). A greater variety of escape tactics, a form of behavioral "aspect diversity" (Rand 1967), presumably reduces the ease with which predators can capture lizard prey.  
Juvenile of the Kalahari lacertid Heliobolus lugubris (left) and oogpister carabid beetle (right). In the Kalahari desert of southern Africa, juvenile lacertid lizards of the species Heliobolus lugubris employ an interesting anti-predator tactic involving deception known as Batesian mimicry (Huey and Pianka 1977b). These defenseless small lizards mimic noxious "Oogpister" beetles (the Afrikaans translates euphemistically as "eye squirter"), which emit pungent acids, aldehydes, and other chemicals when disturbed. Adult H. lugubris lizards are buff-colored and pale red, matching the color of Kalahari sands. Bodies of juveniles are jet black with white spots (juvenile tails are red matching the sand color). Whereas adults walk with a normal tetrapod lizard gait, with their backs undulating from side to side, juveniles walk stiff-legged, with backs arched vertically holding their reddish tails flat against the ground (this makes the tail difficult to detect). When pursued, young H. lugubris abandon their "beetle walk" and dart rapidly for cover, using normal lizard locomotion. As they reach a size of about 45-50 mm from snout to vent (the size of the largest oogpister beetles), these lizards "metamorphose" into the cryptic adult coloration and permanently abandon the stilt walk. The frequency of broken and regenerated tails is lower in juvenile H. lugubris than among closely related lacertids in the same habitats exposed to common predators, suggesting that this beetle mimicry does reduce predatory attacks.  A very important feature of the Australian sandy deserts is the complex burrows of Egernia striata (Pianka and Giles 1982); these nocturnal skinks dig elaborate tunnel systems which are used as diurnal retreats by many species of nocturnal geckos, including Heteronotia binoei, Nephrurus levis, and Rhynchoedura ornata. Egernia excavations are also exploited as refuges from predators and the elements by various diurnal lizards, including Ctenophorus isolepis and Varanus eremius. Large elapid snakes (Pseudechis australis and Pseudonaja nuchalis) are also regularly encountered in these tunnel systems. E. striata burrows are elaborate, with several interconnected openings often as far as a meter apart, and vaguely reminiscent of a tiny rabbit warren. Most of the sand removed from a striata burrow is piled up in a large mound outside one "main" entrance, which usually points south or southwest (Pianka and Giles 1982). Activity of E. striata is strongly seasonal, with 83% of 195 skinks being collected during the summer months of November through February.   Another sympatric, but smaller and more crepuscular, Egernia species, E. inornata, digs a much simpler burrow, consisting of a U-shaped tube with but one arm of the "U" open (this is the sole entrance and only open exit to the burrow); the other arm of the "U" typically stops just below the surface of the ground and is used as an escape hatch by breaking through in an emergency. E. inornata individuals may often have two such burrows 10-20 meters apart. Burrows of inornata are usually about a third of a meter beneath the surface at their deepest spot, and are somewhat shallower than those of striata. The sand removed from inornata burrows is typically spread out in a thin, fan-like, layer radiating away from the entrance (lizards have been observed pushing sand out and smoothing it over with their forefeet and then backing down into their hole). In contrast to striata burrows, this entrance most often faces north or northwest (Pianka and Giles 1982). In addition to being more diurnal than E. striata, Egernia inornata are not as strongly seasonal in activity (only 44% of 131 skinks were collected in the four months from November through February). Thus, E. inornata is appreciably more active during colder weather. Why do the two species construct such different burrows and why do they have such curious compass orientations? Moving all of the sand out of a single opening of an extensive and complex striata burrow system would seem to entail considerable extra energetic expenditure: what could be the counterbalancing benefits? Deep sand is a darker red than surface sand and such tailings give away the positions of burrow entrances, perhaps increasing the liklihood that they will attract undesirable attention (these mounds themselves are sometimes hidden inside Triodia tussocks). Alternatively, the mound itself could serve as a convenient lookout and/or basking platform (the southerly orientation might facilitate the latter function by providing a sloping surface roughly perpendicular to the sun's rays from the north). E. striata individuals are occasionally encountered basking during daylight hours. The north-facing entrances to inornata tunnels are more difficult to explain. One possibility is that a lizard sitting in such an entrance during the day would be exposed to the relatively warm northern sky. Thus interpreted, the interspecific difference between these two Egernia in orientation of their burrows would reflect the observed difference in seasonality and extent of diurnal activity.  References on Parasitism and Antipredator Tactics Arnold, E. N. 1988. Caudal anatomy as a defense. Chapter 3 (pp. 235-274) in C. Gans and R.B. Huey (eds.) Biology of the Reptilia, Volume 16, Ecology B. Defense and Life History. Bromwich, C. R. and J. J. Schall. Infection dynamics of Plasmodium mexicanum, a malarial parasite of lizards. Ecology 67: 1227-1235. Congdon, J. D., L. J. Vitt and W. W. King. 1974. Geckos: adaptive significance and energetics of tail autotomy. Science 184: 1379-1380. Endler, J. A. 1986. Defense against predators. Chapter 8 (pp. 109-134) in Feder, M.E. and G.V. Lauder, eds. 1986. Predator-Prey Relationships. Perspectives and approaches from the study of lower vertebrates. Univ. Chicago Press. Feder, M. E. and G. V. Lauder, eds. 1986. Predator-Prey Relationships. Perspectives and approaches from the study of lower vertebrates. Univ. Chicago Press. Gill, D. E. and B. A. Mock. 1985. Ecological and evolutionary dynamics of parasites: The case of Trypanosoma dicmyctyli in the red-spotted newt Notophlhalmus viridescens. pp. 157-183 in Robinson, D. and R. M. Anderson (eds.) Ecology and Genetics of Host-Parasite Interactions. The Linnean Society of London, Academic Press. Greene, H. W. 1988. Antipredator mechanisms in reptiles. Chapter 1 (pp. 1-152) in C. Gans and R. B. Huey (eds.) Biology of the Reptilia, Volume 16, Ecology B. Defense and Life History. Greene, H. W. and R. W. McDiarmid. 1981. Coral snake mimicry: Does it occur? Science 213: 1207-1212. Huey, R. B. and E. R. Pianka. 1977. Natural selection for juvenile lizards mimicking noxious beetles. Science 195: 201-203. Pianka, E. R. 1970. Comparative autecology of the lizard Cnemidophorus tigris in different parts of its geographic range. Ecology 51: 703-720. Pianka, E. R. and W. F. Giles. 1982. Notes on the biology of two species of nocturnal skinks, Egernia inornata and Egernia striata, in the Great Victoria desert. Western Australian Naturalist 15: 44-49. Pough, F. H. 1988. Mimicry and related phenomena. Chapter 2 (pp. 153-234) in C. Gans and R.B. Huey (eds.) Biology of the Reptilia, Volume 16, Ecology B. Defense and Life History. Rand, A. S. 1964. Inverse relationship between temperature and shyness in the lizard Anolis lineotopus. Ecology 45: 863-864. Rand, A. S. 1967. Predator-prey interactions and the evolution of aspect diversity. Atas do Simposio sobre a Biota Amazonica 5: 73-83. Schall, J. J. 1982. Lizard malaria: Parasite-host ecology. Chapter 5 in R.B. Huey, E.R. Pianka, and T.W. Schoener, (eds.), Lizard ecology: Studies of a model organism (pp. 84-100). Harvard Univ. Press, Cambridge, Mass. Schall, J. J. 1983. Lizard malaria: cost to vertebrate host's reproductive success. Parasitology 87:1-6. Schall, J. J. 1992. Parasite-mediated competition in Anolis lizards. Oecologia 92: 58-64. Schall, J. J. and E. R. Pianka. 1980. Evolution of escape behavior diversity. Amer. Natur. 115: 551-566. Schall, J. J. and G. A. Sarmi. 1987. Malarial parasitism and the behavior of the lizard, Sceloporus occidentalis. Copeia 1987: 84-93. Schall, J. J., A. F. Bennett, and R. W. Putman. 1982. Lizards infected with malaria: physiological and behavioral consequences. Science 217: 1057-1059. Schoener, T. W. 1979. Inferring the properties of predation and other injury-producing agents from injury frequencies. Ecology 60:1110-1115. Smith, S. M. 1975. Innate recognition of coral snake pattern by a possible avian predator. Science 187: 759- 760. Smith, S. M. 1977. Coral-snake pattern recognition and stimulus generalisation by naive great kiskadees (Aves: Tyrannidae). Nature 265: 535-536. Telford, S. R. 1984. Haemoparasites of reptiles. pp. 385-517 in Hoff, G. L., F. L. Frye, and E. R. Jacobson (eds.), Diseases of Amphibians and Reptiles. Plenum. Vitt, L. J. and J.D. Congdon. 1978. Body shape, reproductive effort, and relative clutch mass in lizards: resolution of a paradox. Amer. Natur. 112: 595-608. Vitt, L. J. and W. E. Cooper. 1986. Tail loss, tail color and predator escape in Eumeces (Lacertilia: Scincidae): age-specific differences in costs and benefits. Can. J. Zool 64: 583-592. Vitt, L. J., J. D. Congdon, and N. A. Dickson. 1977. Adaptive strategies and energetics of tail autotomy in lizards. Ecology 58: 326-337. Vitt, L. J. 1992. Mimicry of millipedes and centipedes by elongate terrestrial vertebrates. Research and Exploration 8: 76-95. Back to Herpetology Home Page |
